Characterizations of the LMMH
In this study, we elaborated on the synthesis of LMMH nanoparticles, characterized by a unique core–shell design (Scheme 1). The representative transmission electron microscopy (TEM) image of MnO@MON (MM) nanoparticles, as shown in Fig. 1A, demonstrated that the MM exhibit satisfactory monodispersity with a spherical morphology. Also, the TEM image confirmed the presence of mesoporous pores on the rough surface of the MM, which enhances their functionalization capacity and is essential for their potential application in cargo delivery. To further confirm the core–shell structure of the MM nanoparticles, HAADF imaging and EDX mapping were employed. In these analyses, yellow represents the presence of manganese, blue represents silicon, red represents oxygen, and green represents sulfur (Fig. 1B). The elemental analysis confirmed the effective incorporation of MnO nanoparticles into the core of the organic mesoporous silica matrix. As illustrated in Figure S2 (A, B, C, and D), the hydrated size of LMMH (164.7 ± 18.5 nm) was slightly larger than that of LMM (142.3 ± 7.7 nm) after multiple modifications. As depicted in Fig. 1C, the zeta potential of LMMH was measured at − 14.8 mV, which is higher than MM (− 31.4 mV). This increase is attributed to the amination modification of the mesoporous silicon surface. The drug loading ratio of LT was determined to be 17.93%. In summary, the observed charge reversal and particle size changes during the synthesis process clearly demonstrate that the multifunctional modification of LMMH was successfully implemented according to the pre-designed scheme. The nitrogen adsorption desorption isotherm (Figure S3A) and the corresponding pore size distribution (Figure S3B) indicate that the specific surface area and pore size of LMMH are approximately 534.7 m2/g and 3.1 nm, respectively. Furthermore, it was found that the incorporation of MnO does not affect the morphology of the resulting nanoparticles.
Characterization of the LMMH. A Representative images of TEM and HAADF-STEM of MM. B Elemental mapping images of Mn, Si, O and S elements for MM. C Zeta potential of MnO, MON, MnO@MON (MM), LT, LT@MnO@ MON (LMM) and LT@MnO@MON-HA (LMMH). D Hydrodynamic diameters distributions, corresponding polydispersity index (PDI) and zeta potential of LMMH dispersed in PBS (pH = 7.4) during 7 days at room temperature. E Schematic illustration of the Fenton-like reaction catalyzed by LMMH. F UV–vis absorbance spectra of MB solutions with different reaction buffer. G The remaining percent of MB after different treatments in (F). H UV–vis absorption spectra of MB treated with LMMH in different concentration of H2O2. Data are shown mean ± SD, n = 3
To assess the stability of nanoparticles, LMMH was dispersed in a PBS solution and maintained at room temperature for one week. During this period, daily measurements of the particle size and zeta potential of LMMH were performed to evaluate its stability and distribution in aqueous solutions. As expected, the nanoparticles diameter in the suspension remained relatively constant during 7 days, confirming the stability of LMMH under physiological conditions (Fig. 1D). This stability helps to prolong the blood circulation time of LMMH to enhance the therapeutic effect of LT. Previous studies have indicated that Mn2+ can catalyze endogenous H2O2 to produce toxic ·OH through a Fenton like reaction, a process known as CDT. Due to the release of Mn2+ by MM nanoparticles in acidic environments, we continued to investigated whether LMMH can catalyze endogenous H2O2 in situ and generate ·OH in the TME (Fig. 1E). MB was utilized as a dye indicator to assess ROS generation. The UV–Vis absorbance data shown in Fig. 1F, along with the quantitative analysis in Fig. 1G, demonstrated that MB degradation in the presence of H2O2, HCO3–, and Mn2+. Furthermore, LMMH nanoparticles showed similar effects in H2O2 and HCO3–, suggesting that LMMH has Fenton-like activity similar to that of Mn2+. Additionally, it was observed that the degradation of MB enhanced with increasing H2O2 concentrations (0 to 5 mM) (Fig. 1H).
Microenvironment responsiveness characteristics of LMMH
The response properties of -S–S- bonds enable mesoporous silicon material to specifically react with the reducing microenvironment containing high concentrations of GSH in tumor tissues [35, 36]. To utilize the GSH-responsive properties of materials with -S–S- bonds, a structure incorporating an organic R group and bioactive -S–S- bonds was engineered into MON [34]. This modification allowed the MON to undergo biodegradation in a reducing TME. We further validated this design through in vitro drug release experiments. To assess the cumulative drug release profile of LT, LMMH was transferred to dialysis bags and immersed in PBS solution (pH 7.4) with or without GSH ([GSH] = 5, 10 mM). It was observed that LT was effectively released from the macropores of LMMH. As illustrated in Fig. 2A, in the abesence of GSH, the release of LT was negligible during initial 8 h, followed by a gradually increase over the subsequent 24 h. After a 48-h incubation, the release of LT in GSH-free PBS was approximately 12.78%, suggesting that LMMH effectively prevents premature release of LT under normal physiological conditions. Additionally, the GSH-sensitive release behavior of LMMH under reducing conditions was also examined. LT demonstrated a rapid initial release within the first 4 h at a 5 mM GSH concentration and within the first hour at a 10 mM GSH concentration. Subsequently, the release rate increased over the next 24 h and continued gradually up to 48 h in the presence of GSH. The release profile exhibited a marked sensitivity to GSH, with a significantly higher cumulative release observed at 10 mM GSH compared to 5 mM. Specifically, over a 48-h period, the cumulative release reached approximately 50.48% at 5 mM GSH and 93.06% at 10 mM GSH. These findings indicated that LMMH is sensitive to GSH, with GSH-induced degradation leaking to the break-down of organic mesoporous structure, thereby facilitating the rapid release of the loaded drugs [37].
Biosafety and biodegradability of LMMH in vitro. A The accumulative LT release from LMMH at pH 7.4 with or without GSH (5 mM and10 mM) within 48 h (n = 3). B The accumulative LT release from LMM, LMMH, and LMMH + HAse in PBS (pH 7.4) solution within 48 h (n = 3). C Accumulative Mn release of LMMH in PBS solution with different pH values (7.4, 6.5, and 5.0) at room temperature (n = 3). D The relative GSH level of MnO, MM, and LMMH in PBS solution ([GSH] = 5 mM) during 48 h. E The ability of LMMH to consume GSH ([GSH] = 5 mM) under different concentration conditions (n = 3). F Corresponding average dynamic light scatterings (DLS) diameter of LMMH in GSH (5 mM or 10 mM) (n = 3). G Cell viability of 4T1 cells after being incubated with different concentrations of MnO, H MM for 24 h and 48 h by CCK8 assay (n = 3). I Viabilities of 4T1 cells incubated with LT, LMM, and LMMH (n = 3). The experimental data were presented as mean ± SD. ns: no significance, *P < 0.05, **P < 0.01, ***P < 0.001
The HA coating serves dual functions: it improves the targeting of cancer cells via interaction with the CD44 receptor and provides protection against premature release of LT [38]. As illustrated in Fig. 2B, there was a notable distinction in the LT release profiles between LMM and LMMH. LT was rapidly released from LMM, achieving a cumulative release of 87.47% within 48 h. In contrast, LMMH, which was capped with HA, exhibited a slower and more sustained release of LT, with only 16.84% released in the same period. The HA capping effectively reduces the risk of premature LT release during circulation, thereby minimizing potential adverse effects on healthy tissues. Furthermore, the addition of HAase accelerated the release of LT from LMMH. HAase enzymatically degrades the outer HA layer, facilitating LT release. Consequently, LMMH was capable of delivering a more potent antitumor response, as the Haase-induced rapid release of LT in tumor cells, may lead to a more precise and effective therapeutic outcome. In Fig. 2C, the immersion of LMMH in PBS solutions with pH values of 5.0 and 6.5, result in Mn2⁺ release rates of 49.06% and 20.44%, respectively, over a 48-h period. These findings suggested that the acidic TME facilitated the degradation of LMMH, consequently promoting the release of Mn2⁺. Additionally, as degradation progresses from the exterior to the interior, the release of Mn2⁺ from LMMH was observed to increase over time, demonstrating a time-dependent release pattern. Notably, minimal Mn2⁺ release (5.16% at 48 h) was detected in a neutral PBS environment, indicating that LMMH displayed acid-dependent Mn2⁺ release characteristics and exhibited remarkable stability under physiological conditions. These findings unequivocally showed that LMMH, while maintaining its structural integrity under standard physiological conditions, displayed a sensitivity to degradation within the TME.
To assess the GSH-depletion capacity of LMM, MnO, MM, and LMMH were incubated with 5 mM GSH in PBS for 48 h to simulate a reductive TME. As depicted in Fig. 2D, there was a significant, sequential reduction in GSH levels in the presence of MM and LMMH. This reduction was attributed to the high concentration of disulfide bonds present in the mesoporous silicon materials. After 48 h, the relative GSH levels in the MnO, MM, and LMMH groups were 97.56%, 13.09%, and 27.76%, respectively. The more rapid GSH consumption by MM compared to LMMH, was due to the direct interaction of the disulfide bond-containing shell of MM with GSH. In addition, the initial concentrations of the three nanomaterials (MnO, MM, and LMMH) were maintained consistently throughout the study. During the preparation of LMMH, the encapsulation of the HA shell, result in MM contained a higher proportion of organic mesoporous material and therefore more disulfide bonds for the same mass. Consequently, MM exhibited greater GSH consumption within 48-h period. However, LMMH was still can consume 70% of GSH, thereby maintaining a substantial capacity to modulate the tumor’s reductive microenvironment. And because LMMH combined LT and Mn2+ to achieve synergistic anti-tumor therapy, the therapeutic effect of LMMH at the in vivo level may be better. The absence of disulfide bonds in the MnO structure explained its negligible effect on GSH concentration in the microenvironment. Furthermore, a progressive decrease in the residual GSH levels content correlated with an increase in LMMH in the solution. As depicted in Fig. 2E, at a constant GSH concentration, when the LMMH concentration reached 1640 µg/mL, only 8.82% of the initial GSH remained in the solution. Additionally, the decrease in LMMH particle size after GSH incubation directly confirmed the nanomedicine’s GSH-triggered degradation (Fig. 2F).
Biosafety and enhanced cellular uptake performance
After confirming the nanoparticles’ structure and properties, we tested their cytotoxicity using the CCK-8 assay. Additionally, to thoroughly understand the synergistic antitumor effects of LMMH, a thorough evaluation of the safety of the components used in its fabrication was carried out. As depicted in Fig. 2G, within the experimental concentration range of 0–320 µg/mL, MnO materials demonstrated excellent cell compatibility, with cell viability remaining high at 94.62% and 86.93% after 24 and 48 h of co-incubation with 4T1 cells, respectively. Upon encapsulation of MnO with organic mesoporous silica, the biosafety concentration range was significantly enhanced. In particular, after 24 and 48 h of co-culture with 4T1 cells, cell survival rates remained at 84.08% and 80.04%, respectively, when the MM concentration was 1 mg/mL (Fig. 2H), which showed the favorable biosafety profile of MM, highlighting its potential as a drug carrier.
Furthermore, an evaluation of the drug toxicity of free LT was performed (Fig. 2I). Notably, after a 24 h incubation, the cell viability was found to be around 30% even at a concentration of up to 500 µg/mL. This finding emphasizes that significant inhibition of cell growth occurs only at relatively high concentrations of LT. Despite efforts to enhance its targeting specificity, the therapeutic efficacy remained constrained. This observed low cellular toxicity can be attributed to the limited effective concentration of LT internalized by the cells. In contrast, when tumor cells were incubated with LMM and LMMH, respectively, at a material concentration of 500 µg/mL ([LT] = 100 µg/mL), a pronounced cellular toxicity was evident. This outcome could be due to LMMH’s diverse mechanisms against tumor growth, with improved drug cellular uptake being crucial.
Consequently, an investigation into the targeted cellular uptake of LMMH was subsequently conducted. To facilitate cell localization, LMMH were initially labeled with the FITC and subsequently co-incubated with the 4T1 cells for 0, 2, 4, 8 h. After incubating LMMH with the cells for 2 h, weak green fluorescence was observed within the cells (Fig. 3A). As the incubation time increased, the intensity of the green fluorescence gradually strengthened. Quantitative analysis of the green fluorescence within the cells using flow cytometry revealed that the highest intensity of green fluorescence, indicating the maximum uptake of LMMH, was observed after 8 h of co-incubation with 4T1 cells (Fig. 3B). Subsequently, the endocytosis pathway was investigated using different inhibitors, including chlorpromazine (an endocytotic inhibitor of clathrin-mediated endocytosis), filipin (an inhibitor of caveolae-mediated endocytosis), amiloride (inhibitor of Na+/H+ pump related macropinocytosis) and cytochalasin (an endocytotic inhibitor of macropinocytosis-mediated endocytosis). As shown in Fig. 3C, the endocytosis amount decreased in cells treated with different inhibitors. Notably, at 4 °C, the cellular uptake was significantly reduced. Since reducing the temperature to 4 °C was known to suppress endocytic activities, our findings indicated that LMMH was mainly taken up by cells through endocytosis.
Enhanced cellular uptake and ROS reaction activity. A Representative fluorescence images of 4T1 cells localization at 2, 4 and 8 h after LMMH treatment, scale bar = 20 µm. red channel: phalloidin, blue channel: DAPI. green channel: FITC. B The corresponding average fluorescence intensity values of (A) (n = 5). C Evaluation of endocytic pathways using endocytic pathway inhibitors through flow cytometry (n = 3). D The corresponding ROS fluorescence intensity in different concentration of LT groups via Flow cytometry detection. E CLSM images of cellular ROS against 4T1 cells after treated with different concentration of LT (scale bar = 100 μm)
Glycolysis metabolism intervention and fenton-like reaction activity of LMMH in vitro
Intracellular ROS generation serves as a primary indicator of CDT. Subsequently, we evaluated LMMH’s Fenton-like activity in cells by measuring ·OH generation in 4T1 cells with different treatments. DCFH-DA, which readily crosses the cell membrane, is cleaved by intracellular esterase to form non-fluorescent DCFH. ROS within the cells oxidize DCFH to produce fluorescent DCF. Consequently, 4T1 cells treated with various conditions were labeled with the fluorescent dye and analyzed using confocal microscopy and flow cytometry to measure the levels of ROS. Flow cytometry analysis further confirmed that at an LT concentration of 160 µg/mL, a substantial increase in ROS production was observed, surpassing the effect elicited by free H2O2 (Fig. 3D). And a higher concentration of LT was associated with a progressive increase in the green fluorescence signal, which is indicative of the elevated intracellular ROS (Fig. 3E).
LMMH-treated 4T1 cells, with or without H2O2, exhibited markedly higher fluorescence than PBS and H2O2 controls, as detected by CLSM in Fig. 4A. Conversely, cells co-incubated with MM exhibited minimal ROS levels. To further validate the intracellular Fenton-like reaction activity of LMMH, flow cytometry was employed to quantify ·OH generation (Fig. 4B). Collectively, these findings indicated that LMMH maintains robust Fenton-like reaction activity, catalyzing H2O2 into toxic ·OH. Building on these data, subsequent experiments were conducted to investigate whether LMMH could induce cell apoptosis. Flow cytometry was employed to evaluate cellular apoptosis, and the findings depicted in Fig. 4C indicated that LMMH alone triggered substantial cell apoptosis via being stained with the Annexin-V-FITC/PI, with the inclusion of H2O2 intensifying this apoptotic response. These findings highlight the potential of LMMH as a CDT chemodynamic drug. Quantitative data obtained from flow cytometry analysis exhibited a consistent trend, as depicted in Fig. 4D.
The activated LMMH-mediated Fenton-like reaction activity. A Intracellular ROS levels in 4T1 cells after different treatments (PBS, H2O2, MM, LMMH, or LMMH + H2O2), scale bar = 100 µm. B The corresponding average fluorescence intensity values of (A) (n = 5). C Scatter diagram showing 4T1 cells apoptosis after different treatments by flow cytometry. D Quantified analysis of cell apoptosis ratio in (C) (n = 5). E–G Relative level of (E) G6P, F NADP.+/NADPH ratio, G relative level of LA. The experimental data were presented as mean ± SD, n = 5. ns: no significance, *P < 0.05, **P < 0.01, ***P < 0.001
Branching off from glucose metabolism, the pentose phosphate pathway (PPP) is highly active in many cancers, leading to the generation of substantial amounts of NADPH and ribose-5-phosphate [39, 40]. NADPH acted as a crucial reducing agent in synthetic processes, playing a role in the synthesis and regeneration of GSH, as well as in shielding cancer cells from oxidative stress [41]. Research had shown that depleting NADPH can effectively increase the susceptibility of tumor cells to oxidative stress, thus enhancing the potency of CDT-induced DNA damage [42, 43]. Consequently, in 4T1 cells treated with formulations containing LT, there was a decrease in the hexokinase II (HKII)-catalyzed production of glucose-6-phosphate (G6P), which was involved in both glycolysis and the PPP (Fig. 4E). To assess PPP inhibition, the NADP+/NADPH ratios were examined in cells cultured with various formulations. Notably increased levels were detected in groups exposed to LT, LMM, and LMMH, validating the disruption of NADPH production by LT (Fig. 4F). To assess the inhibition of glycolysis, the concentration of lactate (LA), a byproduct of glycolysis, was measured using a lactate assay kit. As illustrated in Fig. 4G, a considerable decrease in intracellular LA levels was noted after treatment with LT-containing formulations.
The enhanced antitumor immune response induced by LMMH
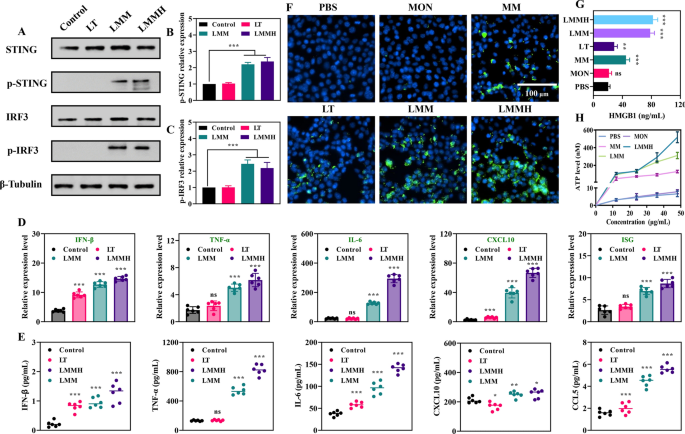
To substantiate the synergistic activation of the STING pathway elicited by LMMH, an investigation into STING pathway activation was conducted via western blot analysis, which assessed cellular levels of p-STING protein, a well-established marker of STING activation (Fig. 5A). When compared to the control and LT groups, DC cells within the LMM and LMMH groups demonstrated a notable elevation in the expression levels of both p-STING (Fig. 5B) and p-IRF3 (Fig. 5C), suggesting that LMMH effectively activated the cGAS-STING pathway, likely due to the release of Mn2+. It should be emphasized that the activation of the cGAS-STING pathway leads to the release of multiple cytokines that can combat tumors by either directly damaging cancer cells or indirectly enhancing anti-tumor immune responses. As depicted in Fig. 5D, the secretion of cytokines (IFN-β, TNF-α, IL-6, CXCL10, and ISG) related to the cGAS-STING pathway by DC in the LMM and LMMH groups was higher than in other groups. Furthermore, quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis revealed that DC cells treated with LMMH exhibited higher relative expression levels of cGAS-STING-related genes (IFN-β, TNF-α, IL-6, CXCL10, and CCL5) compared to those treated with LT and LMM (Fig. 5E). Altogether, these results show that LMMH triggers a coordinated activation of the cGAS-STING pathway, boosting its anti-cancer effects.
Activation of the cGAS-STING pathway in dendritic cells. A Representative western blot analysis of intracellular p-STING expression after varied treatments. B Quantitative protein expression by western blot analysis of p-STING (n = 5), and p-IRF3 (C) (n = 5). D Quantitative real-time PCR measuring the gene expression of IFN-β, TNF-α, IL-6, CXCL10, and ISG (n = 6). E Extracellular concentrations of cGAS-STING-related cytokines (IFN-β, TNF-α, IL-6, CXCL10, and CCL5) in different groups (n = 6). F Immunofluorescence staining of CRT expressions on 4T1 cells surfaces after different treatments (scale bar = 100 μm). G Levels of HMGB1 secretion following different treatments (n = 6). H ATP release in a time-dependent manner following MON, MM, LMM, or LMMH treatment, respectively (n = 6). The experimental data were presented as mean ± SD. ns: no significance, *P < 0.05, **P < 0.01, ***P < 0.001
New studies highlight that activating the STING pathway in some tumor cells can cause immunogenic cell death, promoting an inflammatory response that enhances adaptive anti-tumor immunity [44]. Subsequently, an examination was conducted to assess the occurrence of ICD in 4T1 cells by measuring various biochemical markers, including the cell surface expression of calreticulin (CRT), as well as the release of HMGB1 and ATP. Notably, fluorescent staining revealed pronounced CRT expression on the cell surface following LMMH treatment (Fig. 5F). Among the various treatment groups, those receiving LMM and LMMH exhibited the highest levels of extracellular HMGB1 secretion, indicating effective release of this molecule (Fig. 5G). Additionally, ATP release was observed to be time-dependent in cells treated with MM, LMM, and LMMH (Fig. 5H). Overall, LMMH emerged as a potent inducer of ICD in tumor cells, stimulating the enhanced release of HMGB1 and ATP to elicit adaptive anti-tumor immune responses, while the surface expression of CRT served as a “eat me” signal for antigen-presenting cells.
In vivo antitumor immune activation by LMMH
Encouraged by the initial findings, we then proceeded to assess LMMH’s effects on metabolic interference and immune activation to inhibit 4T1 tumor growth in vitro. A bilateral 4T1 mouse tumor model was developed and treated with different regimens as outlined in Fig. 6A. All experiments were performed in compliance with the ethical and legal standards of the Administration Committee of Experimental Animals in Chongqing Province and were sanctioned by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University (IACUC-SAHCQMU-2023–0066). The analysis of growth rates for both the primary (Fig. 6B) and metastatic tumors (Fig. 6C) indicated that LMMH treatment significantly impeded the growth of both types of tumors. Tumors in the saline or MM-treated groups showed swift expansion, with negligible tumor suppression due to the limited effectiveness of MM (Fig. 6D). On day 21, the average volume of the primary tumor in the LMMH group was recorded at 96 ± 49 mm3, markedly lower than the average volumes in the LMM group (351 ± 119 mm3), LT group (889 ± 174 mm3), and MM group (1277 ± 121 mm3), respectively. The diminished efficacy of free LT was likely due to its rapid metabolic clearance during circulation.
Antitumor effects of LMMH in 4T1 tumor bearing mice. A Schematic description of 4T1 tumor xenograft and therapeutic outcome with LMMH. Individual tumor growth profiles of primary (B) and distant (C) tumor. Primary (D) and distant (E) tumor volume changes of 4T1 tumor-bearing mice after different treatments including saline, MM, LT, LMM, or LMMH for 21 days. F Tumor suppression rate. G Primary tumor weight of collected 4T1 tumors from different groups at Day 21 after various treatments. Data were shown as mean ± S.E.M, n = 6. *P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001
During the LMMH treatment phase, an initial inhibition of distant tumor growth was evident (Fig. 6E), which could be attributed to the Mn2⁺ mediated anti-tumor response. Notably, LMMH demonstrated significantly enhanced anti-tumor efficacy compared to both LT and MM, resulting in substantial tumor growth suppression (Fig. 6F), ultimately achieving a 93.3% tumor inhibition ratio after 21 days. This finding underscored the synergistic effect of combining the Mn-based nanocarrier with LT. Following the treatment period, the mice were euthanized, and their tumor tissues were harvested. The results further indicated that the mean primary tumor weight in the LMMH group was only approximately 22.34% of that in the saline-treated group (Fig. 6G). Although the average tumor weight in the MM group was modestly lower than in the saline-treated group, the therapeutic outcome was less significant than anticipated. This might be due to the inadequate immune response provoked by the pure Mn2+-based nanomaterials, which was not sufficient to effectively suppress tumor growth. However, when LT and MM were co-administered, the combined effects of metabolic interference-induced tumor cell death and cGAS-STING-triggered immune activation maximized tumor growth inhibition. The metabolic interference mediated by LT may alter the secretion of metabolites in tumor cells, further interfering with the physicochemical environment of the tumor microenvironment and sensitizing the anti-tumor immune response induced by Mn2+. Particularly noteworthy was the LMMH group encapsulated with HA, owing to its potent tumor-targeting ability and high drug encapsulation efficiency, this group showed a near absence of metastatic tumors.
Furthermore, ELISA assays demonstrated that the levels of antitumor-associated cytokines, namely IL-6 (Fig. 7A), CXCL10 (Fig. 7B), TNF-α (Fig. 7C), and IFN-γ (Fig. 7D), were significantly elevated in the LMMH group compared to other therapy, suggesting an enhancement of antitumor efficacy. To evaluate systemic immune activation in vivo, T cells were extracted from the spleens of mice that received different treatments for flow cytometry analysis. Specifically, CD8+/CD45+CD3+ T cells and regulatory T cells (Tregs) (identified as CD4+CD25+FOXP3+ T cells) were quantified. Among the tumor-bearing mice, the LMMH treatment group had the highest percentage of splenic CD8+ T cells compared to the other treatment groups (Fig. 7E). This increase in CD8+ T cell population was conducive to augmenting T cell-mediated antitumor immunity. Concurrently, the LMMH group displayed the lowest abundance of Tregs among all treatment groups, indicating a reduction in systemic immunosuppression (Fig. 7F). To explore the potential of activating the cGAS-STING pathway in tumors to enhance immunotherapy efficacy, we analyzed the macrophage populations within tumor tissues using FACS following treatment. Given the immune-adjuvant-like properties of Mn2+, we investigated its impact on tumor immunology. Compared to the untreated group, LMMH-treated tumors exhibited a significant increase in the infiltration of M1 phenotype tumor-associated macrophages (TAMs) (Fig. 7G), while the proportion of M2 phenotype TAMs was markedly decreased (Fig. 7H). Since M2 phenotype TAMs are implicated in immunosuppression, tumors treated with MM, LMM, and LMMH showed higher M1:M2 ratios compared to saline-treated tumors, suggesting that Mn2+ could modify the immunosuppressive TME.
Immune cell analysis in 4T1 tumor-bearing mice. A Intratumoral concentrations of IL 6, B CXCL10, C TNF α, D IFN β in different groups detected by ELISA. E Fluorescence activated cell sorting (FACS) analysis of CD8+ T cells in tumor tissues. F The corresponding quantification of Tregs. FACS analysis of (G) M1-like macrophages (CD80+) and (H) M2-like macrophages (CD206+) in tumor tissues. I Immunofluorescence analysis of CD8+ T cell and CD31+ in tumors tissues after receiving different treatments. Representative (J) Ki67 and (K) TUNEL stained primary tumor slices of 4T1 tumor-bearing mice after the application of different treatments. Scale bar = 100 μm. Data were shown as mean ± SD, n = 6. ns: no significance, *P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001
T cells were the ultimate effectors of anti-tumor immunity. Prior to recognizing and eliminating tumor cells, T cells had to traffic to and infiltrate tumor tissues. A notable increase in CD8+ T cell levels within primary tumor tissues was observed following LMMH treatment (Fig. 7I), likely attributed to the excretion of CXCL10, which promoted CTL trafficking and infiltration. Additionally, immunofluorescence staining for CD31 (red) was employed to assess blood vessel development in primary tumors. The corresponding results indicated that LMMH suppressed breast cancer growth and tumor angiogenesis in vivo (Fig. 7I). Ki67 staining indicated the rate of cell proliferation in tumors, while TUNEL staining identified DNA fragmentation, which is indicative of apoptotic tumor cells. The inhibition of tumor growth was associated with a marked decrease in the proliferation of tumor cells, as demonstrated by Ki-67 staining (Fig. 7J). Notably, LMMH treatment induced the most pronounced tumor cell apoptosis compared to other groups (Fig. 7K). In summary, these results underscore the crucial importance of effective systemic immune activation as a key mechanism responsible for the anti-tumor effectiveness of LMMH. This immune activation was mediated through the STING pathway. In detail, the stimulation of the STING pathway not only induced immunogenic cell death within tumor cells but also promoted the secretion of multiple cytokines, which together intensified anti-tumor immune reactions. Consequently, the delivery of STING agonists via Mn-based carriers emerged as a promising approach for tumor immunotherapy.
To comprehensively assess the biosafety of LMMH, we conducted a rigorous evaluation. Monitoring body weight changes during the treatment period provided a direct indicator of the overall health status of the mice. Our findings indicated that there was no significant change in the body weight of mice across all treatment groups (Fig. 8A), implying the absence of substantial toxicity from the administered interventions. Furthermore, at the endpoint of the therapeutic procedures, we measured the weight of major organs in subcutaneous tumor-bearing mice (Fig. 8B-F), and no abnormalities or discernible changes were observed. Additionally, we evaluated blood biochemical indices following the treatments. H&E staining of organs showed no pathology (Figure S4). Versus the saline control, treated mice showed stable biochemical marker levels (Figure S5), suggesting no systemic toxicity. Thus, the Mn-based nanocarrier demonstrated biocompatibility and degradability, making it suitable for various medical uses. To further develop Mn-based metal immunotherapy into clinical applications, the simplification and automation of LMMH preparation process are issues that we must consider. Although some research on mesoporous silicon is currently in clinical trials, the effectiveness and safety of nanomaterials administered intravenously still cannot meet expectations. In short, component safety, process simplicity, and cost controllability are issues that must be addressed in the clinical application of nanomaterials in the future.