Preparation and characterization of Dex@NBs-TRPC6
Dexamethasone-loaded nanobubbles (Dex@NBs) were prepared using phospholipid materials, including DPPC, DSPC, DSPE-PEG2000, DSPE-PEG2000-biotin, cholesterol, and the GC drug Dex as the primary components, with internally encapsulated inert sulfur hexafluoride (SF6) gas. Subsequently, utilizing the high-affinity biotin-avidin interaction on the surface of Dex@NBs shell, stable surface modification with the targeting TRPC6 antibody was achieved, yielding an ultrasound- responsive nanobubble system, Dex@NBs-TRPC6, with both imaging and therapeutic functionalities (Fig. 1A). As shown in Fig. 1B, the average diameter of Dex@NBs-TRPC6 in solution was 135.71 ± 12.3 nm with a polydispersity index (PDI) of 0.188. The hydrodynamic diameter of Dex@NBs-TRPC6 is a little bit larger than Dex@NBs (108.82 ± 15.8 nm), suggesting that the TRPC6 was modified on the surface of the Dex@NBs. The number concentrations of Dex@NBs-TRPC6 measured using a multi-analyzer were 2.05 × 1010 bubbles/mL.
To further confirm the successful fabrication of Dex@NBs-TRPC6, the structure was observed using Transmission electron microscopy (TEM). As shown in Fig. 1C, Dex@NBs-TRPC6 were spherical with uniform size with the structure of thin lipid monolayer shell and gas core. The distribution mapping of element composition in Fig. 1D and Table S2 (Supporting Information) demonstrated that the Dex@NBs-TRPC6 are mainly composed of carbon (C), nitrogen (N), oxygen (O), fluorine (F), phosphorus (P) and sulfur (S). Among them, C and O are the primary elements of the lipid membrane shell, while F and S are derived from SF6. The N and P elements found on the surface of the lipid membrane are specific to the TRPC6 antibody, confirming the successful assembly of Dex@NBs-TRPC6.
The composition of Dex@NBs-TRPC6 was then analyzed using Fourier-transform infrared (FT-IR) spectroscopy. The infrared absorption spectra for TRPC6, Dex and Dex@NBs-TRPC6 are illustrated in Fig. 1E. Compared with the spectra of Dex, Dex@NBs-TRPC6 exhibited a special C-C double bond stretching vibrational absorption peak at 1680–1640 cm− 1, which is characteristic of the steroidal structure of Dex. And a C-O double bond stretching vibration absorption peak at 1690–1650 cm− 1 was confirmed the successful loading of Dex. Furthermore, Dex@NBs-TRPC6 showed an antisymmetric telescopic vibrational peak of PO4 − 3 at 1320 − 1260 cm− 1 that was attributed to the TRPC6 protein. These results demonstrated that both TRPC6 and Dex were integrated into the Dex@NBs-TRPC6 structure.
Moreover, the in vitro stability of Dex@NBs-TRPC6 was evaluated when stored at 25 °C in phosphate-buffered saline (PBS) (pH = 7.4) and 10% fetal bovine serum (FBS) for 21 days, respectively. Results in Fig. 1F, G revealed no significant changes in size and zeta potential between PBS and FBS groups at the same time points (e.g., Day 1 PBS vs. Day 1 FBS, Day 21 PBS vs. Day 21 FBS) as determined by statistical analysis, showing that Dex@NBs-TRPC6 has excellent stability in vitro. Nanobubble concentration stability was further evaluated. As shown in FigureS1 (Supporting Information), The initial concentration was (1.5 ± 0.3)×109 particles/mL, remaining at (1.3 ± 0.5)×109 particles/mL (89.7% retention) on Day 7 with no significant variation. The encapsulation efficiency of Dex and the loading content of TRPC6 in Dex@NBs-TRPC6 were measured to be 65.21% and 86.05%, respectively.
Preparation and characterization of Dex@NBs-TRPC6. (A) Schematic illustration of the structure of Dex@NBs-TRPC6 and its conjugation with Dex and TRPC6 antibody. (B) Histogram of size distribution of NBs, Dex@NBs and Dex@NBs-TRPC6 obtained by DLS measurements, respectively. (C) Transmission electron microscopy (TEM) morphology characterization of Dex@NBs-TRPC6. Scale bar = 200 nm. (D) Distribution mapping of the element composition of Dex@NBs-TRPC6. (E) FT-IR scanning of lyophilized powder of free Dex, TRPC6, and Dex@NBs-TRPC6. (F) The size distribution and (G) Zeta potential of Dex@NBs-TRPC6 within 21 days in PBS and FBS. (H) In vitro ultrasound imaging performance and (I) quantitative analysis of Dex@NBs-TRPC6 at different concentrations (0, 0.5×109, 1.0×109, 1.5×109, 2.0×109, and 5.0×109 bubbles/mL). (J) In vitro B-mode and contrast-mode ultrasound images performance of Dex@NBs-TRPC6 at different time intervals (0, 5, 10, 15, 20, and 30 min). (K) Quantitative analysis of the mean power in contrast mode and (L) B-mode of different bubble samples (NBs, Dex@NBs and Dex@NBs-TRPC6). (M) In vitro release profiles of Dex from Dex@NBs-TRPC6 under different power ultrasound stimulation (0, 30, 60, 250, and 500 mW/cm2). (N) Quantitative analysis of the bubble breakage rates under different ultrasound conditions
In vitro ultrasound imaging enhancement of Dex@NBs-TRPC6
To assess in vitro ultrasound imaging enhancement, 1 mL sample with varying concentrations of Dex@NBs-TRPC6 was loaded into gel phatom. The nonlinear harmonic ultrasound imaging capabilities of Dex@NBs-TRPC6 were characterized using the high-resolution ultrasound imaging system (Visual Sonics Vevo2100, Canada) with an MS-250 transducer. Two ultrasound imaging modes were used to assess ultrasound imaging enhancement. B-mode imaging primarily reflects structural morphology and baseline echogenicity, while contrast-mode imaging specifically captures dynamic nanobubble oscillation under acoustic pressure, enhancing sensitivity to nanobubble imaging effects. Consequently, both types of nanobubbles exhibited stronger signal intensities in B-mode compared to contrast-mode imaging. As shown in Fig. 1H, compared to degassed pure water, Dex@NBs-TRPC6 sample, attributed to the presence of SF6 gas cores, significantly enhanced ultrasound reflection and scattering to provide excellent contrast enhancement. However, at higher bubble concentrations, the imaging quality diminished due to high concentration bubble interference. According to the contrast enhancement in Fig. 1I of Dex@NBs-TRPC6 at different concentrations, 1.50 × 109/ml was determined to be the optimal concentration for subsequent experiments. Following the establishment of 1.50 × 109/mL as the optimal microbubble density, we further evaluated the differences in in vitro imaging persistence among NBs, Dex@NBs, and Dex@NBs-TRPC6 formulations. As illustrated in Fig. 1J and Figure S2 (Supporting Information), Dex@NBs-TRPC6 group exhibited significantly prolonged signal retention than Dex@NBs group and NBs group. The enhanced retention kinetics were further validated through time-intensity curve regression analysis (Fig. 1K and L), with a decay imaging half-life (t1/2 = 32.7 ± 3.5 min) of Dex@NBs-TRPC6, markedly exceeding that of Dex@NBs (t1/2 = 18.2 ± 2.1 min, 1.8-fold prolongation) and baseline NBs (t1/2 = 11.9 ± 1.6 min, 2.75-fold increase). Notably, the Dex@NBs-TRPC6 exhibited superior structural stability during the initial 10 min, demonstrating a slowly signal decay compared to the NBs group, indicating that the phospholipid-dextran composite shell combined with TRPC6-targeting modification effectively suppressed microbubble fusion and rupture.
Next, leveraging the acoustic response characteristics of the nanobubble platform, different ultrasound power densities were confirmed to optimize conditions for the release of encapsulated Dex from Dex@NBs-TRPC6. The whole design principle was shown in Figure S3A (Supporting Information). UV-visible light scanning curve and standard curve of Dex solution were shown in Figure S3B, C (Supporting Information). As shown in Fig. 1M, the Dex encapsulated in Dex@NBs-TRPC6 without ultrasound stimulation exhibited minimal leakage, with a smooth release profile remaining consistently minimal. The cumulative leakage rates at 1, 8, and 24 h were 12.03%, 19.52%, and 20.90%, respectively. Upon ultrasound power was applied to Dex@NBs-TRPC6 sample, the Dex release was increased and the release efficiency of Dex exhibited a strong correlation with ultrasound intensity, indicating that ultrasound stimulation can effectively enhance the release of Dex from Dex@NBs-TRPC6. At an ultrasound power density of 30 mW/cm², the cumulative release rate of Dex at 24 h was 57.9%, rising to 71.24% at 60 mW/cm² and reaching its maximum of 98.27% at 250 mW/cm². However, at 500 mW/cm², the cumulative release rate dropped marginally to 92.37%. To directly demonstrate ultrasound-induced physical disruption of nanobubbles, TEM imaging was conducted to compare a control group (untreated nanobubbles) and a test group under the same ultrasound condition as the drug release study (1 MHz frequency, 100 Hz pulse frequency, 250 mW/cm2, 1 min). Figure S4 (Supporting Information) revealed that control Dex@NBs-TRPC6 nanobubbles formulation maintained intact spherical structures (average diameter: 136.61 ± 13.17 nm, smooth surface, insert), whereas sonicated Dex@NBs-TRPC6 formulation exhibited collapse or fragmentation, with formulation diameter reduced to 38% of the control (average diameter: 52.43 ± 18.45 nm, insert).
The optimal ultrasound conditions for the release of Dex in vivo were determined using indirect methods. Figure S3D (Supporting Information) showed that after ultrasound intensity stimulation at 250 mW/cm2, the ultrasound imaging signal in mouse kidney decreased most significantly, indicating the highest bubble disruption rate. It was consistent with the in vitro experimental results. Quantitative analysis in Fig. 1N showed that the bubble disruption rate at 250 mW/cm2 reached 50.39%. Therefore, subsequent experiments were conducted with an external ultrasound power density of 250 mW/cm2 as the optimal condition.
In vitro targeting effect of Dex@NBs-TRPC6
Mouse podocyte cell line (MPC) was used to evaluate targeting effect of Dex@NBs-TRPC6 in vitro. Figure 2A, B demonstrated that ADR induced podocyte injury and apoptosis in both dose and time-dependent manner, characterized by reduced expression of podocyte marker podocin and the activation of apoptotic molecule cleaved caspase 3. At the same time, ADR also induced a significant increase in TRPC6 expression of MPC cells. TRPC6 expression level was also demonstrated to be 3.24-fold higher in ADR-induced mouse model than that in control mice (Figure S5 in Supporting Information). Further, TRPC6 expression across different podocytopathies was performed. It was shown in Figure S6 (Supporting Information) that TRPC6 levels in FSGS, IgA nephropathy (IgAN), and MN specimens were significantly elevated compared to controls, with fold change of 2.78, 3.62, and 4.24, respectively. These findings provided the rationale for its selection as our therapeutic target.
The targeting ability of Dex@NBs-TRPC6 was verified in MPC cells. The green DiO dyes labeled Dex@NBs and Dex@NBs-TRPC6 were incubated with MPC cells for different time, respectively. Results in Fig. 2C showed that for Dex@NBs-TRPC6, an obvious speckled distribution of green fluorescence was observed in the cytoplasm after incubation of 12 h and reached a maximum level at 48 h. However, the fluorescence intensity in Dex@NBs group was much weaker than that in the Dex@NBs-TRPC6 group at the corresponding time point. The percentage of DiO stained cells was quantitatively determined using flow cytometer. As shown in Fig. 2D, the DiO positive cell rate displayed a time-dependent manner, both in Dex@NBs group and Dex@NBs-TRPC6 group. After co-incubation for 12 h, the proportion of DiO stained cell in the Dex@NBs-TRPC6 group was 26.06%. It was 8.07-fold higher than that in the Dex@NBs group, which was only 3.23%. At 24 h, 64.15% cells were stained by DiO in Dex@NBs-TRPC6 group, while only 29.47% cells were stained by DiO in Dex@NBs group. After co-incubation for 48 h, the DiO positive rate was reached a saturation degree in Dex@NBs-TRPC6 group, which was still significantly higher than that in Dex@NBs group (75.93%). These results clearly indicated active and efficient target recognition of Dex@NBs-TRPC6 to MPC cells due to the specific TRPC6 expressed on cell membrane of podocytes.
Cell targeting efficiency of Dex@NBs-TRPC6 in vitro. (A) Left: WB analysis of TRPC6, podocin and cleaved caspase 3 activation with different incubation time (0, 12, 24, and 36 h) in a dose of 1 µg/mL ADR treatment. Right: Densitometry analysis. n = 3. (B) Left: WB analysis of TRPC6, podocin and cleaved caspase 3 activation with different doses (0, 0.5, 1.0, and 1.5 µg/mL) of ADR treatment for 24 h. Right: Densitometry analysis. n = 3. (C) In vitro targeting efficiency of Dex@NBs-TRPC6 were observed with fluorescence microscopy after different co-incubation times (0, 8, 12, 24, and 48 h). Nuclei were stained by DAPI. Dex@NBs (as a comparison) and Dex@NBs-TRPC6 were pre-labeled by DiO (green), scale bar = 50 μm. (D) Left: Flow cytometry determined the cell targeting efficiency of Dex@NBs and Dex@NBs-TRPC6 at the same time point as described in figure (C). Right: Quantitative analysis of the flow cytometry results. Data are presented as mean ± SEM, one-way ANOVA, **p < 0.01, Dex@NBs-TRPC6 vs. Dex@NBs group
Evaluation of safety and therapeutic effect of Dex@NBs-TRPC6 in vitro
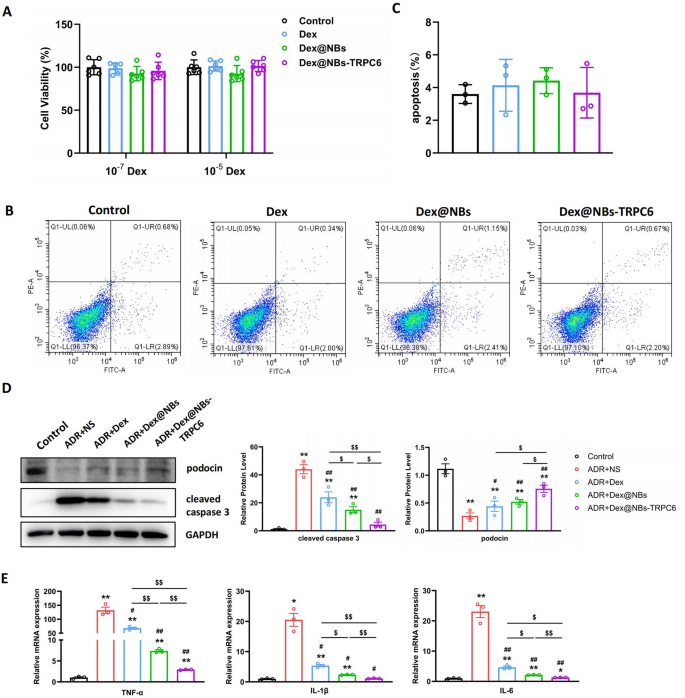
The cytotoxicity of Dex@NBs-TRPC6 on MPC cells was determined using a CCK-8 kit. The cytotoxicity of free Dex, Dex@NBs and Dex@NBs-TRPC6 at a low dose (10− 7 mol/L) and a high dose (10− 5 mol/L) was investigated. Results in Fig. 3A showed that there was no significant cytotoxicity even in a high Dex concentration in each group. Apoptosis analysis in Fig. 3B, C showed that in a dose of 10− 5 mol/L of Dex, free Dex, Dex@NBs and Dex@NBs-TRPC6 exhibited the same apoptotic rate as the control group. It further confirmed that Dex@NBs-TRPC6 was safe to MPC cells. Based on the dose and time gradient results shown in Fig. 2A, B, 1 µg/mL and 24 h were selected for ADR treatment conditions. A dose of 10− 7 mol/L of Dex was used in three Dex-containing groups. As shown in Fig. 3D, three Dex treatments all greatly inhibited ADR-induced apoptosis, presented by activation of cleaved caspase 3. Although cell apoptosis was significantly inhibited in all three Dex-containing therapeutic groups, the targeting treatment of Dex@NBs-TRPC6 was still more effective than free Dex or non-targeting Dex@NBs.
In addition, podocin is also a reactive molecule for Dex treatment. Dex could alleviate the damage to podocytes by stabilizing podocin expression. Results in Fig. 3D showed that ADR intervention significantly lowered the expression of podocin, which was reversed by treatment of free Dex, Dex@NBs and Dex@NBs-TRPC6. Dex@NBs-TRPC6 treated group showed the most effective therapeutic capability.
Furthermore, as shown in Fig. 3E, in ADR-treated MPC cells, all three Dex treatments significantly ameliorated the upregulation of mRNA expression of inflammatory cytokines TNFα, IL-1β, IL-6. Dex@NBs-TRPC6 showed the most prominent effect.
Therefore, all these results indicated that Dex@NBs-TRPC6 provided an excellent podocyte targeting, protective, anti-apoptotic and anti-inflammatory effect in vitro.
Evaluation of safety and therapeutic effect of Dex@NBs-TRPC6 in vitro. (A) Cell viability of MPC cells treated with free Dex, Dex@NBs and Dex@NBs-TRPC6 in dose of 10− 5 and 10− 7 mol/L of Dex. n = 6. (B) Representative images of cell apoptosis determined by flow cytometry in MPC cells treated with free Dex, Dex@NBs and Dex@NBs-TRPC6 in a dose of 10− 5 mol/L of Dex. (C) Statistical analysis of flow cytometry, n = 3. (D) Left: Representative WB images of podocin and cleaved caspase 3 for MPC cells after treated by a dose of 1 µM ADR for 24 h. Normal cells without any treatment was dedicated as control group. Usage dose of Dex was 10− 7 mol/L in free style or in nanobubbles formulation. NS: 0.9% NaCl solution as a vehicle. Right: Densitometry analysis, n = 3. (E) RT-PCR analysis of mRNA expression of TNFα, IL-1β, IL-6 (n = 3). Data are presented as mean ± SEM, one-way ANOVA. *p < 0.05, **p < 0.01, vs. Control group #p < 0.05, ##p < 0.01, vs. ADR + NS group, $p < 0.05, $$p < 0.01 as indicated
In vivo targeting biodistribution of Dex@NBs-TRPC6
We next analyzed the biodistribution of Dex@NBs-TRPC6 in ADR mice model. As illustrated in Fig. 4A, when red fluorescence DiR-labeled Dex@NBs or Dex@NBs-TRPC6 were intravenously injected into ADR model mice, tissues of heart, liver, spleen, lung and kidney were removed at different time points and subjected to the ex vivo optical imaging. Figure 4B, C showed that DiR fluorescence signals were mainly observed in liver and the least in heart at all time points (0.5, 1, 4, 8, and 24 h). For Dex@NBs and Dex@NBs-TRPC6, at 8 h after injection, signals were obviously decreased in liver and increased in lung and kidney. At 24 h after injection, signals were attenuated in lung and kidney and sustained in liver. Although the total variation trend of fluorescence signal was similar in both Dex@NBs and Dex@NBs-TRPC6 injected ADR mice, there was difference of fluorescence signal at every time point between these two groups (Fig. 4D, E). In specific, the kidneys at different time points after injection were compared separately. Figure 4F, G showed that the relative fluorescence signal in kidney of Dex@NBs-TRPC6 group was higher than Dex@NBs group at every time point. Especially at 8 h after injection, the relative fluorescence intensity proportion in kidney of Dex@NBs-TRPC6 group was 2.72 fold higher than Dex@NBs group.
Then, the targeting ability and efficiency of Dex@NBs-TRPC6 in ADR model mice were also verified using a high resolution ultrasound imaging system. First, the maximum blood flow profile of kidney was determined. Then, the ultrasound signal was recorded before injection of nanobubbles and continuously recorded until 150 min after injection. Figure 4H, I showed that the ultrasound contrast signal was significantly stronger in Dex@NBs-TRPC6 group than that in Dex@NBs group at every time point. These biodistribution experiments demonstrated that Dex@NBs-TRPC6 can be efficiently and specifically targeted to the kidneys of the ADR model mice.
In vivo biodistribution of Dex@NBs-TRPC6 in ADR-induced model mice. (A) Schematic representation of fluorescence imaging protocol. The typical fluorescence imaging of Dex@NBs (B) and Dex@NBs-TRPC6 (C) in heart, liver, spleen, lung, and kidney in ADR-induced model mice at different periods of time (0.5, 1, 4, 8, and 24). n = 3. Quantitative analysis of the relative fluorescence intensity of Dex@NBs (D) and Dex@NBs-TRPC6 (E) corresponding to the image (B) and (C), respectively. (F) Comparison of the fluorescence intensities of Dex@NBs and Dex@NBs-TRPC6 in ADR mouse kidney at different time points (0.5, 1, 4, 8, and 24 h). (G) Quantitative analysis of accumulation of Dex@NBs and Dex@NBs-TRPC6 in kidney at different time points, n = 3. (H) Representative kidney ultrasound enhancement images of Dex@NBs and Dex@NBs-TRPC6 in kidney of ADR-induced model mice at different time points (0, 5, 15, and 120 min). Circles in blue line presented kidney area. (I) Quantitative analysis of ultrasound signal intensity of Dex@NBs and Dex@NBs-TRPC6 at different time points after injection. n = 3. Data are presented as mean ± SEM, one-way ANOVA, **p < 0.01, vs. Dex@NBs group. ##p < 0.01, vs.0.5, 1, 4, and 24 h in Dex@NBs group. $$p < 0.01, vs.0.5, 1, 4, and 24 h in Dex@NBs-TRPC6 group
Enhanced renoprotective efficacy of Dex@NBs-TRPC6 in ADR mice
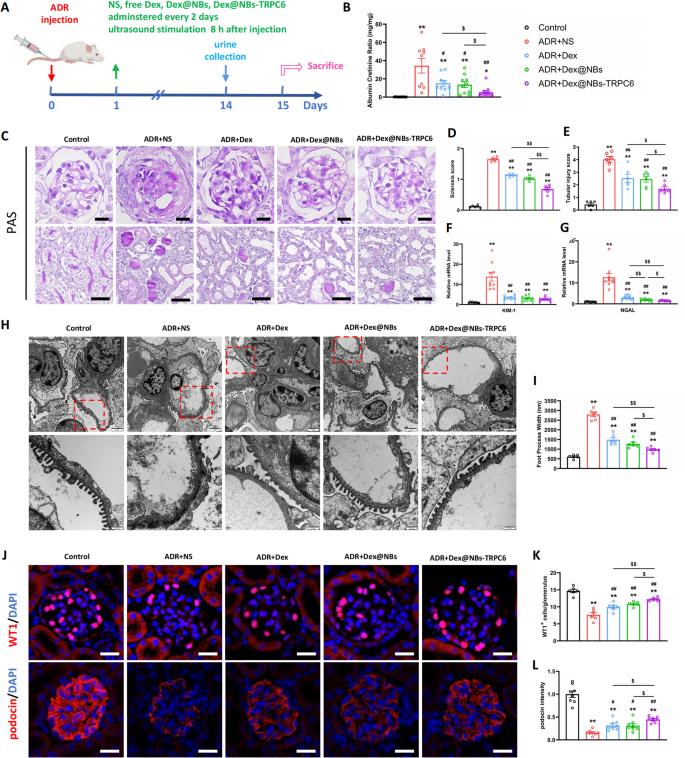
ADR-induced nephropathy model mice were used for in vivo studies. As described in Fig. 5A, 1 day after injection of ADR, mice were treated intravenously with normal saline (NS), free Dex (2 mg/kg), Dex@NBs (loading 1 mg/kg Dex) and Dex@NBs-TRPC6 (loading 1 mg/Kg Dex) every other day until 2 weeks (Day 13) post-ADR injection. 8 h after injection, all mouse kidneys were subjected to ultrasound stimulation for 1 min under 250 mW/cm2. Normal mouse without any treatment was dedicated as control group. Results in Fig. 5B showed that ADR caused a large amount of urine protein. In contrast to free Dex and Dex@NBs treatment, Dex@NBs-TRPC6 treatment caused a more obvious reduction in proteinuria. Dex@NBs-TRPC6 reduced proteinuria to 16.1% of the non-treated ADR group, demonstrating significantly superior efficacy compared to free Dex group (44.1%) and the non-targeted Dex@NBs group (39.5%). ADR nephropathy was characterized by severe lesions in both glomerular and tubular compartments. PAS staining in upper row of Figs. 5C showed segmental glomerulosclerosis in ADR group. Compared with free Dex or Dex@NBs treatment, there was a more significant reduction in glomerular lesions in mice treated by Dex@NBs-TRPC6 (Figs. 5D). In the lower row of Figs. 5C, ADR also induced severe tubular injury, including loss of brush border, tubular dilation, cast formation, and cell lysis. As shown in Fig. 5E, compared with free Dex or Dex@NBs treatment, when treated by Dex@NBs-TRPC6, tubular injury was significantly ameliorated.
Furthermore, the mRNA expression of tubular damage markers Kim-1 and NGAL was measured by RT-PCR. Kim-1 is significantly upregulated in proximal tubular epithelial cells upon injury, serving as a sensitive biomarker for AKI. NGAL is secreted by renal tubules and neutrophils and is acting as an early signal of tubular damage. Figure 5F, G showed that the mRNA levels of both Kim-1 and NGAL were extremely increased in ADR mice. After treatment by free Dex, Dex@NBs or Dex@NBs-TRPC6, the mRNA levels of Kim-1 and NGAL were all remarkably decreased. In particular, Dex@NBs-TRPC6 treatment showed more effective decrease of NGAL expression than free Dex or Dex@NBs.
Podocyte injury was also morphologically evaluated by TEM. As shown in Fig. 5H, control healthy mice displayed an orderly arrangement of processed foot and endothelial cells and no thickening of the basement membrane. However, the ADR mice showed severe diffuse fusion of foot processes. After treatment by free Dex, Dex@NBs or Dex@NBs-TRPC6, fusion of foot processes was obviously ameliorated. Further statistical analysis in Fig. 5I showed that the podocyte foot process width in Dex@NBs-TRPC6 group was significantly smaller than that in free Dex group or Dex@NBs group.
We further confirmed that Dex@NBs-TRPC6 improved the therapeutic efficacy of Dex on podocytes based on immunofluorescent analysis of WT-1 and podocin, two specific biomarkers of podocytes. As shown in Fig. 5J, ADR mice showed significantly decreased levels of WT1 and podocin. Dex administration partially reversed the damage effect of ADR. Quantitative analysis in Fig. 5K, L demonstrated that Dex@NBs-TRPC6 was the most effective in rescuing levels of either WT1 or podocin.
Altogether, all these data supported the more beneficial effects of Dex@NBs-TRPC6 to attenuate proteinuria, glomerular lesions, and tubule-interstitial injury.
In vivo therapeutic effects of Dex@NBs-TRPC6. (A) Schematic diagram of in vivo treatment and therapy protocol. Normal mouse without any treatment was dedicated as control group. (B) Albuminuria (urine albumin-to-creatinine ratio) was determined in control group, ADR + NS group and three Dex-treated ADR groups. n = 9–10 mice per group. (C) Representative kidney images of PAS staining. Upper: glomerular morphology, scale bar = 20 μm; Lower: tubule-interstitial morphology, scale bar = 50 μm. (D) Quantitative analysis of glomerulosclerosis severity. (E) Quantitative analysis of tubular injury. (F) RT-PCR analysis of mRNA expression of Kim-1. (G) RT-PCR analysis of mRNA expression of NGAL. n = 9. (H) Representative images of TEM of glomerular basement membrane. Top: scale bar = 2 μm, Bottom: scale bar = 500 nm. (I) Quantitative analysis of foot process width. n = 6 mice per group in all histological analysis. (J) Upper: immunofluorescence staining of WT1 expressed in nuclei of mouse podocytes, scale bar = 20 μm. Lower: immunofluorescence staining of podocin expressed along the glomerular basement membrane, scale bar = 20 μm. (K) Quantitative analysis of WT1 positive cell numbers per glomerulus, n = 6. (L) Quantitative analysis of fluorescence intensity of podocin, n = 6. Data are presented as mean ± SEM, one-way ANOVA. *p < 0.05, **p < 0.01, vs. Control group, #p < 0.05, ##p < 0.01, vs. ADR + NS group, $p < 0.05, $$p < 0.01 as indicated
Enhanced anti-apoptotic, anti-inflammatory and anti-fibrosis efficacy of Dex@NBs-TRPC6 in ADR mice
To further unlock the protective effect of Dex@NBs-TRPC6, assays of anti-apoptosis and anti-inflammation were performed. TUNEL assay in Fig. 6A, B revealed that ADR intervention increased cell apoptosis, while Dex treatment in three groups suppressed ADR-induced apoptosis. Dex@NBs-TRPC6 treatment was the most effective. In line with results of TUNEL assay, Dex@NBs-TRPC6 showed strongest inhibition against ADR-induced cleaved caspase 3 activation (Fig. 6C).
Furthermore, the efficacy of Dex@NBs-TRPC6 treatment in reducing renal inflammation was examined. ADR intervention significantly upregulated the protein levels of IL-1β and IL-6 both in the serum (Fig. 6D) and kidney tissue (Fig. 6E). Compared with free Dex and Dex@NBs treatment, after treated by Dex@NBs-TRPC6, both IL-1β and IL-6 were significantly downregulated. Then, the mRNA expression of several inflammatory mediators in the kidney tissue were determined. As shown in Fig. 6F, the proinflammatory factors such as IL-1β, IL-6, TNFα and MCP1 were significantly upregulated in ADR group. After treated by free Dex, Dex@NBs or Dex@NBs-TRPC6, all these proinflammatory factors were downregulated. The efficacy of Dex@NBs-TRPC6 was the most remarkable. Besides, the anti-inflammatory factor IL-10 was evidently decreased in ADR group, while increased in three Dex-treated groups. Dex@NBs-TRPC6 showed better efficacy than Dex@NBs.
In addition, immunohistochemical staining of F4/80 and Ly6G was performed to analyze the infiltration of macrophages and neutrophils in ADR mouse kidneys, respectively. The upper row of Fig. 6G showed that a large number of F4/80-positive macrophages were infiltrated in the kidneys of ADR mice. After treatment with free Dex, Dex@NBs or Dex@NBs-TRPC6 for 2 weeks, results in Fig. 6H showed that macrophage infiltration was significantly reduced. The Dex@NBs-TRPC6 treatment group demonstrated the best effect. Immunohistochemical staining of Ly6G (lower row of Fig. 6G) displayed the same tendency. ADR intervention induced severe neutrophil infiltration. After treatment with free Dex, Dex@NBs or Dex@NBs-TRPC6, neutrophil infiltration was obviously reduced. Especially, for Dex@NBs-TRPC6-treated group, the number of neutrophils was almost the same as that in the normal control group (Fig. 6I). These results demonstrated that Dex@NBs-TRPC6 had a more potent ability to reduce the infiltration and expression of local inflammatory mediators in the kidney, and may play a role in in-situ immunosuppression of the kidney.
Masson staining was performed to explore the anti-fibrosis efficacy of Dex@NBs-TRPC6 in ADR mice. The segmental glomerulosclerosis and interstitial fibrosis were clearly observed in ADR mice as shown in Fig. 6J. It was significantly ameliorated in mice after treatment with free Dex, Dex@NBs or Dex@NBs-TRPC6. Compared with free Dex and Dex@NBs groups, these fibrotic changes were improved most obviously in Dex@NBs-TRPC6-treated group. Statistical analysis of the Masson-positive area in Fig. 6K further illustrated this phenomenon. The anti-fibrosis effect of Dex@NBs-TRPC6 was further confirmed by RT-PCR and WB analysis. As shown in Fig. 6L, the mRNA levels of the fibrosis factor fibronectin (FN) and α-SMA were significant upregulated in ADR mouse kidney. However, FN and α-SMA expression was remarkably inhibited after treatment with free Dex, Dex@NBs or Dex@NBs-TRPC6. Effects of Dex@NBs-TRPC6 were more evident than free Dex and Dex@NBs. Results of WB in Fig. 6M were consistent with that of RT-PCR. The protein expression levels of FN and α-SMA in the Dex@NBs-TRPC6 treatment group were almost similar to those in the control group. Collectively, Dex@NBs-TRPC6 can effectively suppress ADR-induce renal apoptosis, inflammation and fibrosis.
Dex@NBs-TRPC6 reduced apoptosis, inflammation, and fibrosis in ADR mice. (A) Apoptosis cells in kidney tissue were measured by TUNEL staining, Scale bar = 50 μm. (B) Quantitative assessment of TUNEL positive cells, n = 6. (C) Left: Representative WB of cleaved caspase 3 in kidney tissue. Right: Densitometry analysis of cleaved caspase 3, n = 8. (D) IL-1β and IL-6 levels in mouse serum were measured by ELISA, n = 6. (E) IL-1β and IL-6 levels in renal tissue were measured by ELISA, n = 9. (F) RT-PCR measurement of mRNA levels of inflammatory cytokines in kidney tissue including IL-1β, IL-6, TNFα, MCP1 and IL-10, n = 9. (G) Immunohistochemical staining of macrophages (upper) and neutrophils (lower) in mouse kidney tissue, scale bar = 50 μm. (H) Quantitative analysis of macrophages, n = 6. (I) Quantitative analysis of neutrophils, n = 6. (J) Representative images of Masson staining. Upper: glomerular morphology, scale bar = 20 μm; Lower: tubule-interstitial morphology, scale bar = 50 μm. (K) Quantitative analysis of tubule-interstitial fibrosis, n = 6. (L) RT-PCR measurement of mRNA level of FN and α-SMA in mouse kidney tissues, n = 9. (M) Left: Protein expression levels of FN and α-SMA in mouse kidney were determined by Western Blot. Right: Densitometric analysis of proteins levels of FN and α-SMA, n = 8. Data are presented as mean ± SEM, one-way ANOVA, **p < 0.01, vs. Control group. ##p < 0.01, #p < 0.05, vs. ADR + NS group. $$p < 0.01, $p < 0.05 as indicated
Biosafety evaluation of Dex@NBs-TRPC6 in ADR mice
To determine whether Dex@NBs-TRPC6 was safe in vivo or it could reduce side effects of systemic Dex, we investigated the impacts of Dex@NBs-TRPC6 on normal indexes such as body weight and blood glucose, as well as biochemical indexes including liver function, kidney function, lipid metabolism and serum albumin. As shown in Fig. 7A, for ADR mice with no treatment, body weight of the mice dropped prominently at the first 4 days, then gradually recovered later, reaching its maximum value on the 8th day, and then gradually declined again. Variation in body weights of Dex-treated groups was similar to that in ADR group. There was no significant difference between ADR group and any of the three Dex therapeutic groups on each time point. Neither difference was found among three Dex-treated groups.
For blood glucose, it was shown in Fig. 7B that no significant different changes were found in each group between Day 0 and Day 15 after ADR injection. There were also no differences among control, ADR + NS and three Dex therapeutic groups on Day 0 or on Day 15, respectively. Figures 7C showed that kidney function suffered significant damage after ADR intervention. Dex treatment significantly reduced serum creatinine (SCr) level. However, there was no difference among these three Dex therapeutic groups. Dex treatment also significantly reduced level of blood urea nitrogen (BUN). Compared to free Dex, Dex@NBs-TRPC6 treatment worked better. No hepatotoxic injury was observed in ADR mice or after treatment with free Dex, Dex@NBs or Dex@NBs-TRPC6 for 2 weeks. In addition, levels of urea acid (UA) were significantly increased in ADR mice and decreased to almost normal level in free Dex, Dex@NBs or Dex@NBs-TRPC6 treated mice. Changes in serum albumin corresponded to changes in urinary ACR (Fig. 5B). Serum albumin was evidently reduced in ADR mice and partially recovered after treatment of free Dex, Dex@NBs or Dex@NBs-TRPC6. Dex@NBs-TRPC6 showed more superior recovery capability than Dex@NBs. In addition, Dex@NBs-TRPC6 achieved the same effect in raising serum albumin level as free Dex, even at a half dosage of Dex. Levels of triglyceride (TG) and total cholesterol (CHO) were both apparently increased in ADR mice. All Dex treatment groups failed to improve TG upregulation, but inhibited CHO production to almost normal condition. However, there was no difference among the three treatment groups.
Furthermore, mRNA expression of gluconeogenic markers PCK1 and G6PC and glycolytic marker HK2 in liver were determined. As shown in Fig. 7D, PCK1 and G6PC were significantly reduced in ADR mice and upregulated to an abnormal high level when treated with free Dex. However, although levels of PCK1 and G6PC were still higher than normal control group, Dex@NBs or Dex@NBs-TRPC6 treated groups showed significantly lower levels than that in free Dex treated group. The reason may be that when Dex was delivered by nanobubble system, the accumulation of Dex in the liver was reduced. Meanwhile, compared to Dex@NBs, Dex@NBs-TRPC6 treatment brought levels of PCK1 and G6PC more closely to normal level because of more enhanced delivery of Dex to kidney and corresponding less delivery to liver. The glycolytic gene HK2 was dramatically upregulated in ADR mice and suppressed obviously in free Dex, Dex@NBs or Dex@NBs-TRPC6 treated mice. However, no differences were found among the three groups.
At last, pathological changes were observed by H&E staining of heart, liver, spleen and lung in each group. The results in Figure S8 showed that none of the treatments induced obvious pathological alteration in heart, liver, spleen and lung tissues. Therefore, these findings indicated that Dex@NBs-TRPC6 was a safe and reliable nano delivery platform for treatment of ADR-induced nephropathy with the least systemic side effects, including abnormally increased gluconeogenesis, a common side effect of Dex.
Side-effect evaluation of Dex@NBs-TRPC6 in ADR mice. (A) Body weights were measured every 2 days during therapeutic period. (B) Levels of blood glucose were measured using blood glucose test strips. (C) Serum levels of SCr, BUN, ALT, AST, UA, ALB, TG and CHO were measured by an automatic biochemical analyzer. (D) RT-PCR analysis of gluconeogenic genes PCK1 and G6PC, and glycolytic gene HK2 in livers. n = 9–10 in A-D assays. Data are presented as mean ± SEM, one-way ANOVA, **p < 0.01, *p < 0.05, vs. Control group. ##p < 0.01, #p < 0.05, vs. ADR + NS group. $$p < 0.01, $p < 0.05 as indicated