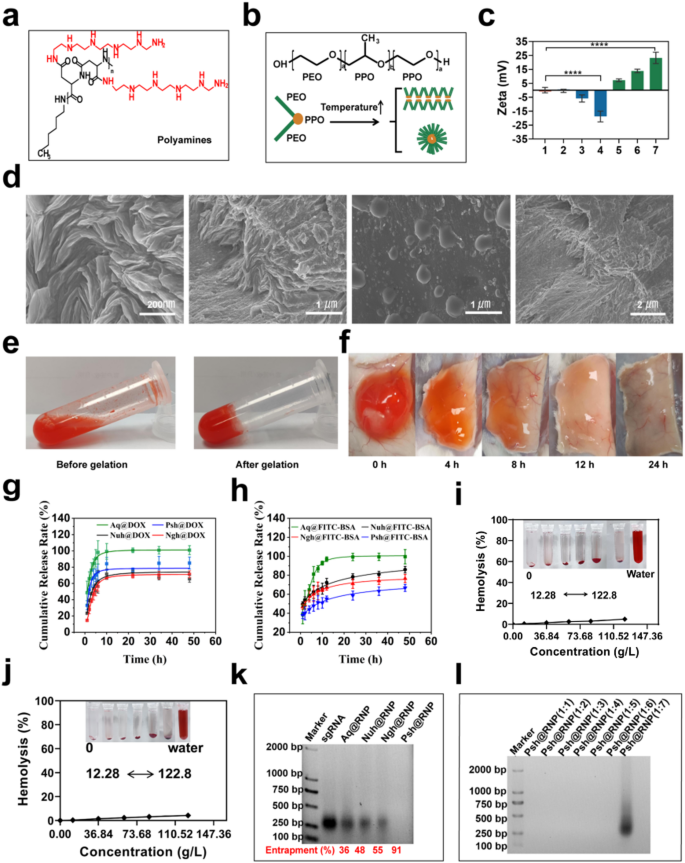
Preparation of hydrogels with different electrical properties
Polyamines (Fig. 2a) were synthesized using N-carboxyanhydride polymerization of L-benzyl aspartate (Fig. S1a), followed by amination of the side chain with N-amine substituents bearing pentaethylenehexamine repeats, as we described in methodology (Fig. S2a). Polyamines were characterized using 1H-NMR (Fig. S1b & S2b). P407 and P188 with a triblock structure (PE0-PPO-PEO) were used as the basic materials (Fig. 2b). Hydrogels with different ratios of P407 and P188 were prepared using the cold-solution method. Characterization of gelation time, gelation temperature and injectability of hydrogels of different hydrogels by syringe injection methods (Fig. S3). We found that aqueous solution with 20.81% of P407 and 3.46% of P188 was injectable and has the most suitable gelation time (40 s) at 37 °C to make Nuh (Table. S3) with a neutral zeta potential. Psh were prepared by adding 0.1% polyamines into Nuh to gain a positive zeta potential, and Ngh were prepared by adding 0.15% HA into Nuh to gain a negtive zeta potential (Fig. 2c). We also prepared drug-loaded hydrogels, including Psh@RNP, Psh@DOX, Psh@DOX@RNP, Nuh@RNP, Nuh@DOX, Nuh@DOX@RNP, Ngh@RNP, Ngh@DOX, and Ngh@DOX@RNP, to do the following experiments. The morphology of Psh was observed by scanning electron microscopy (SEM, Apreo 2, OPTON, Beijing). Typically, materials are ready for observation by the use of freeze-drying. Drying method is employed to prepare samples since this hydrogel must gelling at 37 °C. This leads to the loss of water molecules from the hydrogel, resulting in the collapse of its voids. Microscopic observations at various magnifications reveal that the dried hydrogel’s truncated surface exhibits abundant folds, suggesting that the hydrogel originally possessed a dense void structure before dehydration and that drug loading was achieved through pore adsorption. (Fig. 2d). When the temperature changed from 4 °C to 37 °C, Psh@DOX gelled from a fluid state into a solid state, shrinking to compress its pores and expel the encapsulated drug(Fig. 2e). We subcutaneously injected 100 µL Psh@DOX solution into mice, it was observed that the hydrogel gelled into a subspherical semisolid within 10 min under the skin of mice. Over time, the subcutaneous Psh@DOX gradually disappeared to the point where it was not observed (Fig. 2f). After we studied the in vitro release behavior of Psh@DOX and Psh@FITC-BSA (Fig. S4). Compared with DOX solution (Aq@DOX), DOX in Psh@DOX, Nuh@DOX, and Ngh@DOX released much slower and the release curves of the three groups were similar. Differently, 66.7% of FITC-BSA released from Psh@FITC-BSA after 48 h, while it was 85.9% for Nuh@DOX and 76.6% for Ngh@DOX (Fig. 2g&h). This phenomenon shows that Psh is suitable for the sustained release of protein drugs. Next, we performed hemolysis assay to study the toxicity of hydrogels. The result showed that the hemolysis of Psh, Nuh and Ngh was neglectable. Even at 122.85 g/L, the hemolysis of Psh is less than 5%, indicating that Psh was safe and can be used for subcutaneous injection (Fig. 2i&j & Fig. S5).
We synthesized Cas9 protein and sgRNA in our lab. First, we designed primers to construct pET-28a-Cas9-2NLS-6His recombinant plasmid (Table S1 & Fig. S6). The plasmid were transformed to E.coli BL21 (DE3) competent cells for Cas9 expression and purification (Fig. S7). Second, we designed three sgRNA targeting the YB-1 gene. Primers for the sgRNA sequences were shown in Supplementary Table 2. Three sgRNA sequences with the T7 promoter were obtained by RT-PCR and transcription (Table S2 & Fig. S8). Cas9 protein and sgRNA were incubated at 37 °C for 15 min to obtain RNP. The loading capacity of the three hydrogels was then investigated using gel retardation assay. Using free sgRNA as a reference, Fig. 2k demonstrates that Psh (91%) had the highest RNP loading capacity, which was 1.7 times higher than that of Ngh (55%), and 1.9 times higher than that of Nuh (48%). We further verified the effect of different molar ratios of RNP on the loading performance of Psh, and Fig. 2l showed that Psh can encapsulate RNP with molar ratios 1:1 to 1:6 (Cas9: sgRNA).
Preparation, characterization and comparison of three hydrogels. (a) Structures of polyamines utilized in the study. (b) Schematic representation of micelle formation by Poloxamer at elevated temperatures. (c) Zeta potential of different hydrogels. Column 1, Nuh; Column 2–4, Nuh@0.05%HA, Nuh@0.1%HA, Nuh@0.15%HA; Column 5–7, Nuh@0.05%PA, Nuh@0.1%PA, Nuh@0.15%PA. (d) SEM photographs of the Psh. (e) Psh@DOX is a fluxible liquid at 4 °C and a gel state at 37 °C. (f) Subcutaneous status of Psh@DOX at different time points. (g) In vitro release of Psh@DOX. (h) In vitro release of Psh@FITC-BSA. (i) Evaluation of haemolysis at different concentrations of Nuh. (j) Evaluation of haemolysis at different concentrations of Psh. (k) 2% agarose gel image of RNP-loaded hydrogel preparations. sgRNA was used to determine the encapsulation rate of RNP in hydrogels. (l) 2% agarose gel image of Psh@RNP. Data are presented as the mean ± s.d. (n = 3 biological replicates per group)
Cellular uptake efficiency and in vitro anti-tumor efficacy
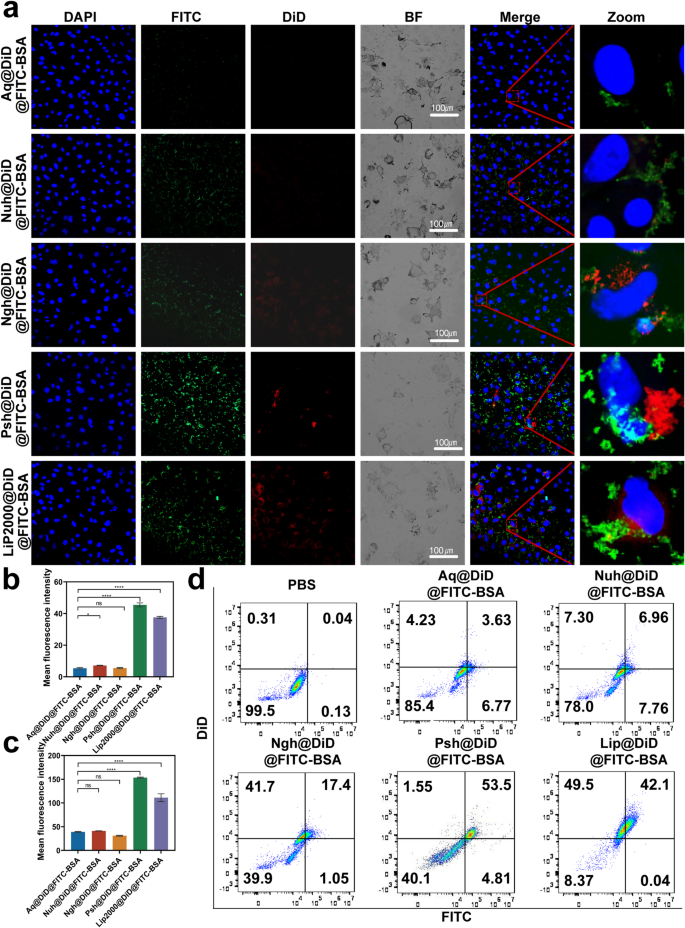
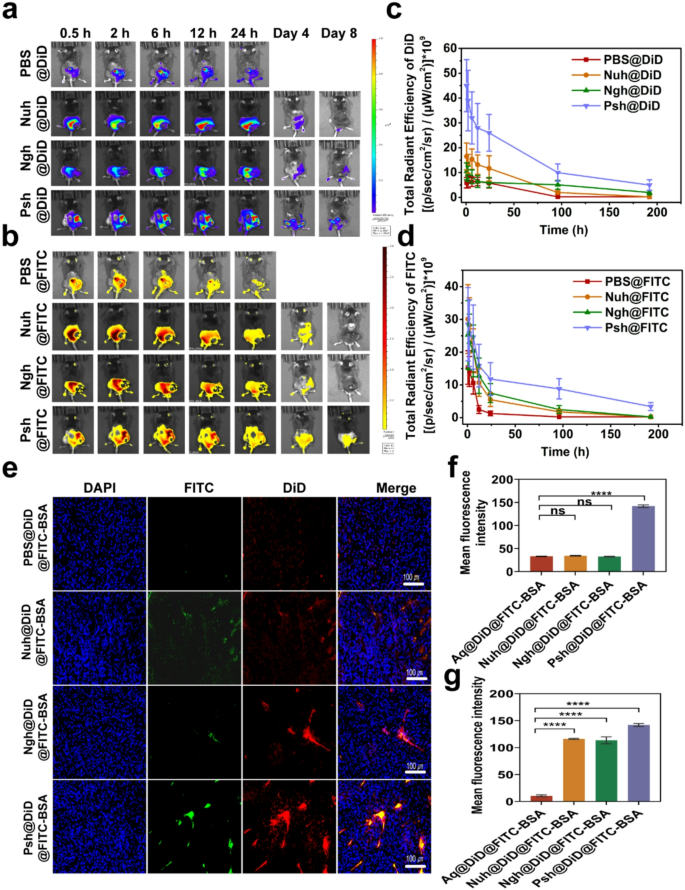
To examine the effect of the developed hydrogel on the cellular uptake of chemotherapeutics and macromolecular drugs, we prepared Aq@DiD@FITC-BSA, Nuh@DiD@FITC-BSA, Ngh@DiD@FITC-BSA, Psh@DiD@FITC-BSA and Lip2000@DiD@FITC-BSA and administrated to B16F10 cells. For visualization purposes, we used DiD instead of DOX and FITC-BSA instead of Cas9. The result of confocal microscopy (Fig. 3a-c) showed that the fluorescence intensity of cells treated with Psh@DiD@FITC-BSA was stronger than that of Aq@DiD@FITC-BSA, Nuh@DiD@FITC-BSA, and Ngh@DiD@FITC-BSA. The difference of fluorescence intensity between Psh@DiD@FITC-BSA and Lip2000@DiD@FITC-BSA was not significant. After 6 h incubation, the percentage of DiD and FITC-BSA positive cells in Psh@DiD@FITC-BSA treated group was 53.5% detected by flow cytometry, whereas it was 3.63% for Aq@DiD@FITC-BSA, 6.96% for Nuh@DiD@FITC-BSA, and 17.4% for Ngh@DiD@FITC-BSA (Fig. 3d). These results proved that Psh mediated high cellular uptake efficiency, and it was suitable as carriers for both DOX and RNP.
Cellular uptake of DiD and FITC mediated by three types of hydrogels. (a) Confocal microscope images of B16F10 cells after 6 h incubation with Aq@DiD@FITC-BSA, Nuh@DiD@FITC-BSA, Ngh@DiD@FITC-BSA, Psh@DiD@FITC-BSA and Lip2000@DiD@FITC-BSA. Cell nucleus was counterstained with 4,6-diamino-2-phenyl indole (DAPI) (blue). Quantitative analysis of (b) DiD and (c) FITC in B16F10 cells. (d) Endocytosis of Aq@DiD@FITC-BSA, Nuh@DiD@FITC-BSA, Ngh@DiD@FITC-BSA, Psh@DiD@FITC-BSA or Lip2000@DiD@FITC-BSA by B16F10 cells, detected by flow cytometry
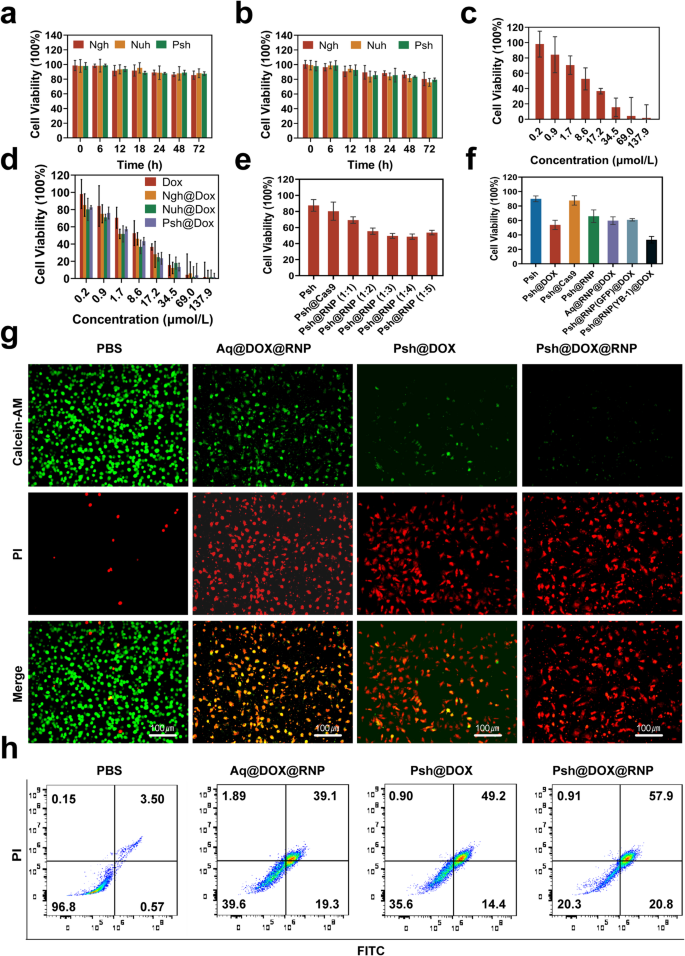
The cytotoxicity of hydrogels was measured in B16F10 and 293T cells by MTT assay. B16F10 and 293T cells were incubated in Nuh, Ngh, and Psh. Cell viability remained stable (~ 85%) even after 72 h of incubation, demonstrating that the blank hydrogel carriers were non-toxic to B16F10 cells and exhibited high biocompatibility. (Fig. 4a&b). Using MTT tests, we further examined how drug-loaded hydrogels inhibited B16F10 cells. Results show that the Psh@DOX displayed much higher cytotoxicity against B16F10 cells with the lowest IC50 value (7.049 µM), which was 0.18-fold lower than that of Ngh@DOX (8.552 µM) and lower than Aq@DOX (10.63 µM) (Fig. 4c&d). To verify the optimal ratios of Cas9/sgRNA, Psh loaded with different molar ratios of RNP were prepared. It was demonstrated by MTT experiments that the inhibition rate of B16F10 increased gradually with the increase of the molar ratio of Cas9/sgRNA until the ratio reached 1:3. When the ratio reaches 1:4, the viability of the cell is instead enhanced, indicating that too high a proportion of sgRNA is instead detrimental to gene editing (Fig. 4e). Thus, we chose 1:3 to do the next experiments. The inhibition rates of blank Psh, Psh@DOX, Psh@Cas9, Psh@RNP, Aq@DOX@RNP, Psh@DOX@RNP(GFP), and Psh@DOX@RNP on B16F10 cells were compared. As shown in Fig. 4f, the cells incubated with Psh@DOX and Psh@RNP exhibited significant lower viability (53.87% and 65.92%) than incubated with blank Psh and Psh@Cas9 (89.92% and 87.72%), suggesting the antiproliferative capacity of Psh@DOX and Psh@RNP. Comparable to the Psh@DOX group (53.87%), the cell survival rate in the Psh@DOX@RNP(GFP) group (60.92%) was higher than that of the Psh@DOX@RNP group (33.28%). It indicated that RNP(GFP) had no evident B16F10 cells cytotoxicity compared to RNP. Psh@RNP suppressed YB-1 gene and prevented cell proliferation. Subsequently, the live-dead cell assay was performed to prove the high therapeutic efficacy of Psh@DOX@RNP (Fig. 4g). After 72 h treatment, the percentage of live B16F10 cells treated with Psh@DOX@RNP was 20.3%, prominently lower than those of PBS (96.8%), Aq@DOX@RNP (39.6%), and Psh@DOX (35.6%) (Fig. 4h).
Pharmaceutical effect of Psh@DOX@RNP on tumor cell B16F10 and non-tumor cell 293T. (a) The toxicity of Psh, Ngh and Nuh on 293T cells at different incubation time. (b) The toxicity of Psh, Ngh and Nuh on B16F10 cells at different incubation time. The viability of B16F10 cells after being treated by (c) free DOX; (d) Ngh@DOX, Nuh@DOX, Psh@DOX; (e) Psh, Psh@Cas9, Psh@RNP (Cas9/sgRNA at molar mass ratio of 1:1, 1:2, 1:3, 1:4, 1:5) and (f) Psh, Psh@DOX, Psh@Cas9, Psh@RNP, Aq@DOX@RNP, Psh@DOX@RNP(GFP), Psh@DOX@RNP. (g) Fluorescence microscopy images of PI (red) and calcein AM (green) cells after different treatments. (h) Apoptosis induced by various treatments to B16F10 cells using flow cytometry. Data are presented as the mean ± s.d. (n = 3 biological replicates per group)
Effective genome editing by Psh@RNP
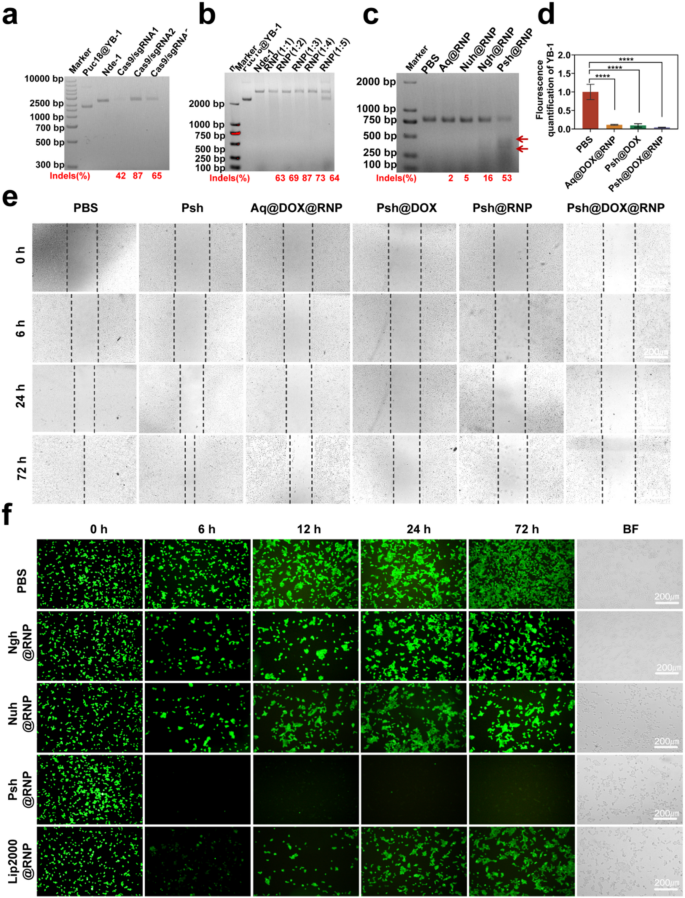
We constructed the PUC18-YB-1 plasmid by design correspondent primers (Table S1&Fig. S9), and the ability of RNP to cleave targeting genes in PUC18-YB-1 plasmid was verified in vitro to screen an optimal sgRNA sequence. As shown in Fig. 5a and Supplementary Fig. S10, all the three sgRNA had a certain endonuclease activity and targetedly edited the YB-1 gene. We chose sgRNA-2 to do the next experiments for its highest endonuclease activity. We also performed double-stranded nucleic acid cleavage assays and verified that Cas9/sgRNA of 1:3 was the optimal molar ratio (Fig. 5b). Following this, an assay utilizing T7EI was conducted to ascertain the efficacy of Psh@RNP-induced genomic insertions or deletions (indels). This endonuclease, which possesses the capability to identify and cleave mismatched DNA sequences, served as a tool to quantify the mutation frequency at the target locus. Specifically, after a 48-hour incubation period, Psh@RNP was observed to elicit double-strand breaks at predetermined genomic loci within B16F10 cells. Following Psh@RNP treatment, a 53% indels frequency could be reached in the targeted locus of YB-1, according to the cleavage bands and quantitative measurement of the intensity of the 2% agarose gel electrophoresis bands using imagej, while almost no mutation frequency could be seen in other groups (Fig. 5c). Q-PCR also proved the knockdown of the YB-1 gene (Fig. 5d).
MT1-MMP gene promotes cancer cell metastasis, and relevant studies have shown that knockdown of the YB-1 gene is accompanied by a decrease in MTI-MMP expression, thereby inhibiting cancer cell migration. Here, we assessed the inhibitory effect of Psh@DOX@RNP by measuring the migration of B16F10 cell in vitro using the wound-healing assay. After 72 h incubation, PBS treated cells repopulated the injured area, and Psh, Aq@DOX@RNP, Psh@DOX, Psh@RNP, Psh@DOX@RNP treated cells were inhibited by about 20%, 50%, 70%, 80%, and 90% respectively (Fig. 5e). The Psh@DOX@RNP had strongest migratory inhibitory effect on B16F10 cells. This result further demonstrates that the YB-1 gene may have been knocked out.
Next, we determined the gene editing efficiency at the GFP gene locus of GFP-293T cell line containing a single copy of the reporter gene GFP and persistently expressing GFP protein. GFP-293T cell line was constructed, and the RNP system targeting the GFP gene was synthesized (Table S2). Gene editing efficiency was qualified using confocal microscopy and quantified using ImageJ software (Fig. 5f). Treatment with Psh@RNP(GFP) converted 67% of the GFP-positive cells to GFP-negative cells, while it was 14% for Ngh@RNP(GFP), 12% for Nuh@RNP(GFP), and 32% for Lipo2000@RNP(GFP), indicating high tumor cell uptake and efficient GFP knocked down by Psh. Collectively, the Psh@RNP could deliver both RNP(YB-1) and RNP(GFP) into B16F10 cells and GFP-293T cells with effective intracellular endo/lysosomal escaping and RNP unpacking, and finally achieved efficient gene editing in vitro.
In vitro gene editing efficiency of Psh@DOX@RNP. (a) Endonuclease activity of RNPs (composed of different sgRNAs) on PUC18@YB-1 plasmid in vitro. (b) Endonuclease activity of RNP (Cas9/sgRNA at molar mass ratio of 1:1, 1:2, 1:3, 1:4, 1:5) on PUC18@YB-1 plasmid in vitro. (c) T7EI assay of B16F10 cells treated with PBS, Aq@RNP, Nuh@RNP, Ngh@RNP and Psh@RNP. (d) The mRNA level of YB-1 was analysed by qRT-PCR. Data are presented as the mean ± s.d. (e) Representative images of the wound healing assays in B16F10 cells from 0 h to 72 h. (f) Fluorescence images of 293T-GFP cells treated with different preparations [PBS, Ngh@RNP(GFP), Nuh@RNP(GFP), Psh@RNP(GFP), Lip2000@RNP(GFP)]. (n = 3 biological replicates per group)
In vivo targeting ability
Hydrogels were loaded with near-infrared dye DiD and FITC-BSA to investigate the distribution of different hydrogels in melanoma-bearing mice. The DiD fluorescence were obtained by IVIS spectrum at different time points. As exhibited in Fig. 6a&b, after hydrogels were intratumorally administrated to mice, the fluorescence of DiD was found to be localized in the tumor area invariably. At 24 h post-injection, three hydrogel groups showed a stronger fluorescence intensity at the tumor site than a solution of free DiD and FITC-BSA, suggesting that hydrogels greatly facilitate the accumulation and slow release of biomolecules. DiD and FITC-BSA accumulation of three types of hydrogels in tumors remained high for 4 days. Eight days post administration, the fluorescence intensity decreased in hydrogel groups (Psh@DiD@FITC-BSA, Ngh@DiD@FITC-BSA, and Nuh@DiD@FITC-BSA) due to metabolism. However, the residual fluorescence intensity of the Psh@DiD@FITC-BSA group remained higher than that of Ngh@DiD@FITC-BSA and Nuh@DiD@FITC-BSA. Mice in the PBS group died after the fourth day of administration, thus no data are presented in Fig. 6c&d. After the IVIS spectrum observation, the mice were dissected and the tumor tissues were made into sections. As shown in Fig. 6e, much more DiD and FITC-BSA fluorescence signal existed in Psh@DiD@FITC-BSA treated group compared with that of Ngh@DiD@FITC-BSA, and Nuh@DiD@FITC-BSA, which further implied polyamides modification helped hydrogels to accumulate in tumor. DiD and FITC-BSA fluorescence intensity of sections was quantified by ImageJ (NIH, USA). As the statistics shown in Fig. 6f&g, DiD and FITC-BSA fluorescence intensity and proportion in Psh@DiD@FITC-BSA treated tumor were 4.3 times and 1.3 times higher than Ngh@DiD@FITC-BSA, 4.1 times and 1.2 times higher than Nuh@DiD@FITC-BSA, indicating that polyamides modification could help protein drug and small molecule drugs to accumulate in tumor. Since genome editing happened in DNA level, the permanent change would be brought once the editing was successful. So, local delivery of CRISPR/Cas9 RNP to tumor tissue is favorable. To investigate the in vivo distribution and safety of the hydrogel, IVIS spectral imaging was performed to capture the biodistribution of DiD and FITC-BSA in mouse organs at 2 h and 24 h post-injection (Fig. S11). Notably, no detectable fluorescence signals were observed in the organs, further confirming the biosafety of the hydrogel.
Sustained release and accumulation of RNP and DOX delivered by Psh in melanoma mouse models. In vivo fluorescence imaging of (a) DiD and (b) FITC in mice at different time points after intratumoral injection of PBS@DiD@FITC-BSA, Nuh@DiD@FITC-BSA, Ngh@DiD@FITC-BSA, or Psh@DiD@FITC-BSA. Quantification of (c) DiD and (d) FITC in mice by in vivo fluorescence imaging. (e) In vivo fluorescence images of isolated tumors from mice after 8 days injection. Cell nucleus was counterstained with DAPI (blue). (f, g) Quantitative analysis of drug accumulation in tumors. Data are presented as the mean ± s.d. (n = 3 biological replicates per group)
In vivo anti-tumor analysis
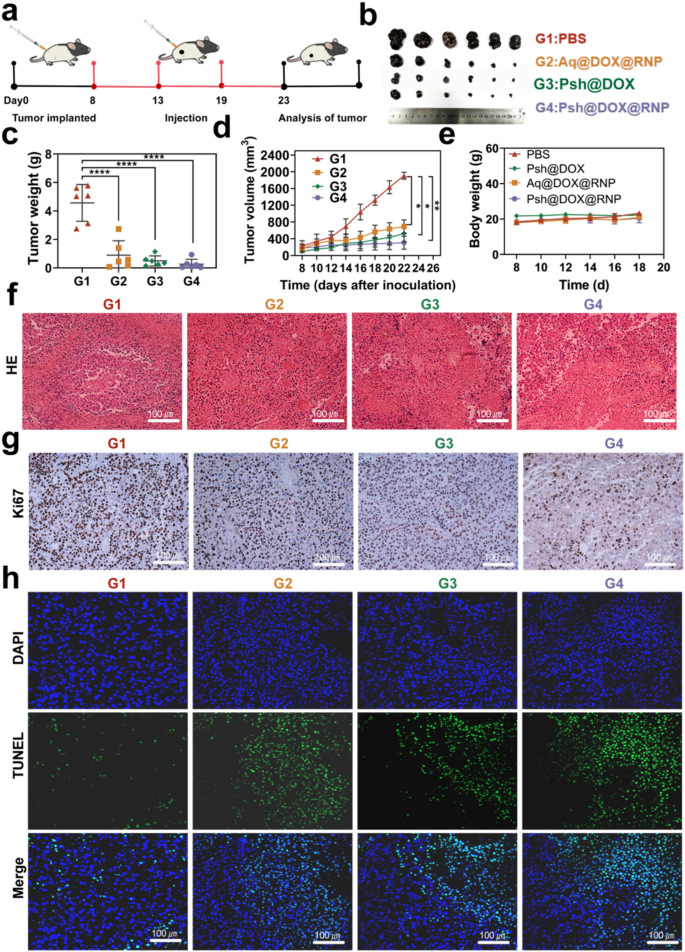
To evaluate in vivo anti-tumor effects, C57BL/6 melanoma model was established as described above, and tumor bearing mice were randomly divided into four groups. PBS, Psh@DOX@RNP, Aq@DOX@RNP, or Psh@DOX was intratumorally injected into B16F10 tumor bearing mice every 5 days, respectively. The treatment scheme was presented in Fig. 7a. Tumor was measured with vernier calipers to monitor tumor growth. As shown in Fig. 7b, the volume of mice treated with Psh@DOX@RNP, Aq@DOX@RNP, Psh@DOX were significantly smaller than PBS group, and the smallest average tumor in the Psh@DOX@RNP group. As shown in Fig. 7c&d, the group treated with Psh@DOX exhibited a rising pattern of tumor progression, attributable to the absence of in vivo YB-1 disruption. It is noteworthy that Psh@DOX@RNP demonstrated a substantial capacity to inhibit tumor deterioration in comparison to the PBS control. Specifically, the tumor growth inhibition rate achieved was approximately 98.4%. Furthermore, Psh@DOX@RNP exhibited superior tumor alleviation compared to Aq@DOX@RNP, owing to its higher accumulation and cellular internalization of DOX and RNP. This enhanced efficacy was facilitated by Psh, which contributed to improved nuclear targeting and accelerated formation of the genome editing complex. During the treatment period, body weight changes were also monitored. The body weight of tumor-bearing mice tended to be stable (Fig. 7e). This phenomenon suggested that it did not elicit systemic toxicity. The H&E staining of isolated tumors after 18 days treatment was shown in Fig. 7f. Obviously, comparing the Psh@DOX@RNP therapy group to the other groups, it is evident that their tumor necrosis area is significantly larger and exhibits the most nuclear shrinkage, fragmentation, and absence. The results confirmed that disrupting YB-1 significantly inhibited tumor proliferation. Notably, upon treatment with Psh@DOX@RNP, the fewest Ki67-positive tumor cells (stained brown) were observed in the tumor tissues, as compared to other groups (Fig. 7g). Additionally, the terminal deoxynucleotidyl transferase – mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay revealed that Psh@DOX@RNP treatment induced the most potent apoptotic effect in tumor tissues when compared to the other groups (Fig. 7h&Fig. S12). Collectively, these findings underscore the synergistic impact of DOX, RNP, and Psh on melanoma.
In vivo anti-tumor efficiency of Psh@DOX@RNP. (a) Treatment scheme. (b) Photographs of tumor dissected from C57BL/6 mice treated with PBS, Aq@DOX@RNP, Psh@DOX, or Psh@DOX@RNP. (c) Tumor weight of the mice. (d) Tumor growth curve of the mice after different treatments. (e) Body weight of C57BL/6 mice after treatment. (f) H&E stained sections and (g) Ki67 Immunohistochemical staining of isolated tumors. (h) TUNEL analysis in tumor sections after different treatments. Data are presented as the mean ± s.d. (n = 6 biological replicates per group)
In vivo gene disruption analysis
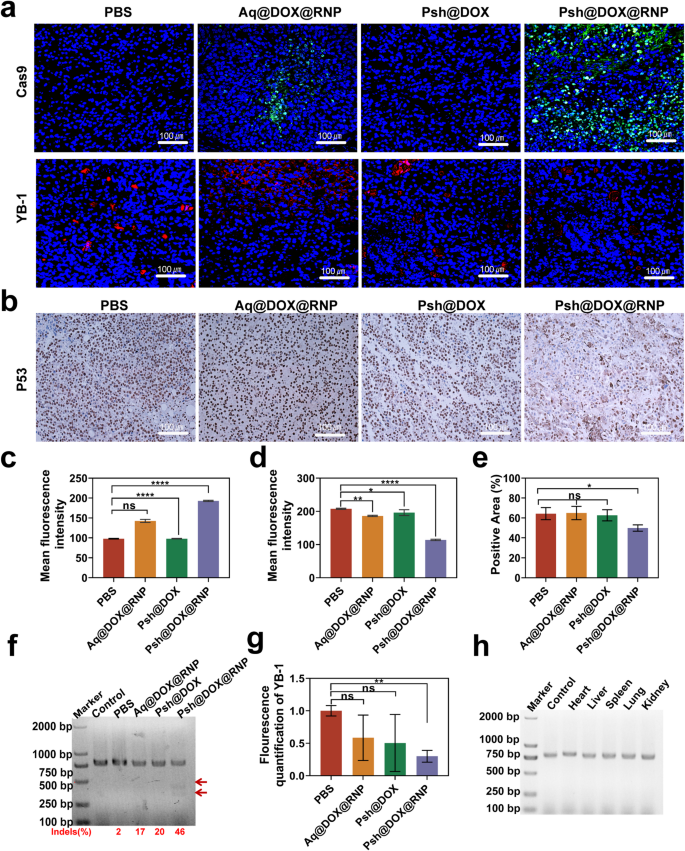
We analyzed the tumor tissue by immunofluorescence and immunohistochemistry at the end of the treatment (Fig. 8a&b). The Cas9 accumulation in tumor tissues treated with Psh@DOX@RNP were about 1.4-fold higher than that of Aq@DOX@RNP (Fig. 8c). As shown in Fig. 8d, the YB-1 fluorescence intensity in the Psh@DOX@RNP group was 45% of the PBS group, indicating that Psh@DOX@RNP successfully delivered RNP into tumor cell and the YB-1 gene were knocked down by Psh@DOX@RNP. While losing its tumor-suppressive function, mutant p53 protein gains novel oncogenic activities through gain-of-function (GOF) mechanisms [42]. Therefore, we investigated the expression of P53 in tumor tissues using immunohistochemical analysis. We found that the Psh@DOX@RNP group had less P53 expression compared to the other three groups (Fig. 8e), which further demonstrated the potential of YB-1 as a therapeutic target for melanoma.
To delve deeper into the mechanism underlying the potent tumor inhibitory capabilities of Psh@DOX@RNP, the in vivo efficacy of YB-1 gene disruption was further validated through the application of the T7EI assay. Consistent with the in vitro results, Psh@DOX@RNP treatment resulted in evident cleavage of YB-1 gene in the tumor tissue with a high gene mutation frequency of 46% (indels%), which was not observed in PBS, Aq@DOX@RNP, and Psh@DOX group (Fig. 8f). Q-PCR of tumor tissue further validated the down-regulation of YB-1 gene expression (Fig. 8g). The assessment of off-targeting editing, immunogenicity, and safety is paramount in CRISPR/Cas9 delivery systems, as these factors can potentially lead to vector clearance and therapeutic failure. Following the administration of Psh@DOX@RNP to mice, a T7EI assay was conducted on potential off-target sites in major organs, namely the heart, liver, spleen, lung, and kidney. As illustrated in Fig. 8h, no discernible cleavage bands were observed, suggesting that Psh@DOX@RNP did not elicit genome editing in these normal organs, thereby mitigating the risk of undesired off-target effects.
In vivo gene editing efficiency of Psh@DOX@RNP. (a) Immunofluorescence analysis of Cas9 (green), YB-1(red) expression. (b) Immunohistochemical analysis of P53 expression in tumor tissue sections. Quantitative analysis of (c) Cas9 protein and (d) YB-1 expression. (e) Quantitative analysis of the number of P53-positive cells. (f) T7EI assay of the isolated tumor tissue. (g) The mRNA of YB-1 in tumor tissues quantified by qRT-PCR. (h) T7EI assay of the isolated heart, liver, spleen, lung and kidney. Data are presented as the mean ± s.d. (n = 6 biological replicates per group)
In vivo safety evaluation
As previously mentioned, the T7EI assay results on off-target locations in vital organs such the heart, liver, spleen, lung, and kidney of treated mice showed that the Psh@DOX@RNP therapy method was safe for biological systems (Fig. 8h). Moreover, a complete blood count (CBC) was performed on treated mice to investigate the safety of different treatments. As shown in Fig. S13, the administration of hydrogels had negligible effect on the number of blood cells. The H&E staining of isolated heart, liver, spleen, lung and kidney after 18 days treatment were shown in Fig. S14. No significant cellular infiltration was observed, demonstrating that this treatment modality has no significant toxic side effects on major organs. Considering the little off-target effect, high safety and low immunogenicity, Psh@DOX@RNP was suitable for in vivo application.