Engineering and characterization of Klotho-overexpression MSCs
The Klotho expression was insufficient in MSC-derived sEV (0.19 µg in 100 µg sEV). To load Klotho into sEV, we first designed and constructed a recombinant lentivirus, then cultured human iPSC-derived MSCs transfected with the recombinant lentivirus of LV-EF1a-3XFlag-Klotho-P2A-EGFP-PGK-Puro-WPRE (LV-KL-MSC) or LV-EF1a-3XFlag-P2A-EGFP-PGK-Puro-WPRE (LV-MSC) (Fig. 1A). The MSCs infected with lentiviruses were GFP-positive (Fig. 1B). We found that the frequencies of GFP+ MSCs were 74% (Fig. 1C) and the mRNA levels of Klotho were significantly increased in LV-KL-MSC (Fig. 1D). Western blot results showed that Klotho protein was overexpressed in MSCs (Fig. 1E). In addition, we found that Klotho overexpression did not affect the expression of MSC markers, including positive (CD73, CD90, and CD105) and negative (CD14, CD19, CD34, CD45, and HLA-DR) markers [34, 35] (Fig. 1F). Therefore, we successfully built a steady cell line by transfecting MSCs with a lentivirus to achieve overexpression of Klotho.
Klotho-overexpressed mesenchymal stem cell preparation. (A) Schematic diagram for the preparation of Klotho-overexpressed MSCs. (B) Detection of green fluorescent protein (GFP) in purified MSCs by means of fluorescent microscopy. Original magnification ×400. (C) Flow cytometry analyses showed that 74% MSCs were GFP (FITC) positive. (D) mRNA levels of Klotho were determined using quantitative real-time PCR (RT-PCR). (E) Klotho protein was overexpressed in MSCs as determined using western blot. (F) Flow cytometry analyses showed that the overexpression of Klotho could not change MSC maker expression (Red, MSCs labelled with antibody; Blue, blank). In all plots, mean with SD are shown. ****p < 0.0001 by unpaired t-test
Isolation and characterization of Klotho-sEV
We isolated sEV from the cell culture CDPF medium using anion-exchange chromatography (Fig. 2A). As determined by nanoparticle tracking analysis, the mean diameters of sEV (163.5 nm) and klotho-sEV (166.7 nm) were comparable, both with a sharp peak at approximately 150 nm diameter (Fig. 2B). sEV/Klotho-sEV with a diameter of less than 200 nm were confirmed by transmission electron microscopy, which revealed the characteristic lipid bilayer (Fig. 2C). Using western blot, we further confirmed that sEV and Klotho-sEV were positive for the conventional sEV markers CD9, CD63, CD81, ALIX, and TSG101, and negative for Calnexin and GM130 (Fig. 2D). As expected, the Klotho protein was successfully loaded into the sEV (Fig. 2E). We further confirmed that Klotho was mostly located on the membrane of the sEV using NanoFCM (Fig. 2F). The levels of Klotho in Klotho-sEV were further analyzed using ELISA, and we identified that the levels of Klotho were 2.15 µg in 100 µg Klotho-sEV. In summary, encapsulation of Klotho in sEV did not affect their characteristics in terms of size, morphology, and conventional markers.
The characterization of Klotho-sEV. (A) Schematic diagram of the isolation of small extracellular vesicles (sEV)/Klotho-sEV using anion-exchange chromatography (AEC). (B) Representative results of the sEV/Klotho-sEV nanoparticle tracking analyses. (C) Transmission electron microscopy images of sEV/Klotho-sEV (scale bar, 200 nm). Original magnification ×59,000. (D) sEV/Klotho-sEV were positive for CD9, CD63, CD81, and ALIX and negative for Calnexin and GM130, as determined using western blotting. (E) Klotho protein levels in sEV/Klotho-sEV were determined using western blotting. (F) Klotho localization was determined using NanoFCM
Klotho-sEV ameliorated rhabdomyolysis-induced AKI
Myoglobin plays a vital role in rhabdomyolysis-induced AKI [28, 29, 36]. To explore the effects of Klotho-sEV on myoglobin-induced cell apoptosis, we successfully developed an in-vitro cell model using 4 mg/mL ferrous myoglobin. Klotho-sEV pre-treatment stably ameliorated myoglobin-induced tubular cell apoptosis, and increased live cell frequencies (Supplementary Fig. 2). Collectively, these data revealed that Klotho-sEV attenuate myoglobin-induced apoptosis in HK2 cells.
To further explore the potential effects of Klotho-sEV on rhabdomyolysis-induced AKI, we developed a murine model by intramuscular injection of 8 mL/kg of a 50% hypertonic glycerol solution into the inferior hind limbs (Fig. 3A). Creatinine and urea are recognized kidney function biomarkers. We found that the intramuscular injection of hypertonic glycerol sharply increased the levels of creatinine and urea in the blood, revealing that the model was successfully developed. Mice injected with Klotho-sEV showed a notable reduction in creatinine and urea levels on Day 3, whereas the effects of sEV seemed unstable (Fig. 3B and C). To further confirm the effects of Klotho-sEV on AKI, the kidney sections were histologically evaluated. Histological evaluation of the vehicle group revealed casts and necrosis of the tubular epithelial cells. Compared with the vehicle group, mice that received Klotho-sEV treatment showed a significantly decreased number of casts and necrotic tubules. No significant difference was observed between the sEV treatment and vehicle groups, as determined by HE staining (Fig. 3D). This was confirmed by PAS staining (Fig. 3E).
Klotho-sEV significantly alleviated rhabdomyolysis-induced AKI. (A) Schematic of the development of rhabdomyolysis-induced AKI in mice. (B) Serum creatinine levels in mice subjected to different treatments. (C) Urea levels in the serum of mice subjected to different treatments. (D) Representative micrographs of hematoxylin-eosin (HE) staining of kidneys of mice with different treatments on Day 3 after damage. Original magnification ×200. (E) Representative micrographs of Periodic Acid-Schiff (PAS) staining of kidneys of mice with different treatments on Day 3 after damage. Original magnification ×200. In all plots, mean with SD are shown. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by one-way ANOVA
The regenerative effects of Klotho-sEV were confirmed using PCNA staining. Compared with PBS and sEV treatments, Klotho-sEV treatment promoted renal cell proliferation (Fig. 4A). We further analyzed the effects of Klotho-sEV on renal damage and inflammatory markers at the mRNA level. In particular, AKI mice showed up-regulated expression of renal injury markers including PAI, NGAL, and SOX9. Administration of sEV slightly decreased the expression of injury markers, whereas Klotho-sEV administration significantly down-regulated the expression of these injury markers (Fig. 4B). In addition, the renal tissue of AKI mice showed a significant upregulation of pro-inflammatory cytokines including interleukin-1beta (IL-1β), IL-6 and tumor necrosis factor alpha (TNF-α). In contrast, Klotho-sEV treatment significantly reduced the expression of pro-inflammatory cytokines (Fig. 4C).
Klotho-sEV significantly promoted proliferation and reduced injury and inflammatory marker expression. (A) Immunohistochemical staining of proliferating cell nuclear antigen (PCNA) in renal tissue. Original magnification ×400. (B) The mRNA levels of PAI, NGAL, SOX9 in the kidney were determined using RT-PCR. (C) The mRNA levels of IL-1β, IL-6, TNF-α in the kidneys were determined using RT-PCR. Means with SD are shown in all the plots. *p < 0.05, **p < 0.01, ***p < 0.001 by one-way ANOVA
In summary, our findings demonstrated the therapeutic and regenerative effects of Klotho-sEV in rhabdomyolysis-induced AKI.
The treatment of Klotho-sEV made the proteins changed in the kidneys
To elucidate the potential molecular mechanisms of Klotho-sEV in the treatment of AKI, the total proteins were isolated from renal tissues 3 d after hypertonic glycerol treatment and subjected to 4D-DIA proteomics. A total of 10,970 proteins were identified in all the renal tissues (Fig. 5A). Compared to the healthy control group, there were 1009 DEPs (296 with upregulation and 713 with downregulation) in the vehicle group, 739 DEPs (221 with upregulation and 518 with downregulation) in the sEV group, and 690 DEPs (250 with upregulation and 440 with downregulation) in the Klotho-sEV group. Compared to the vehicle group, there were 31 DEPs (11 with upregulation and 20 with downregulation) in the sEV group, 168 DEPs (132 with upregulation and 36 with downregulation) in the Klotho-sEV group. The proteins in the control group were clearly different from those in the other three groups (Vehicle, sEV, and Klotho-sEV) (Fig. 5B). DEPs in each comparison (Control vs. Vehicle, Vehicle vs. Klotho-sEV, Vehicle vs. sEV vs. Klotho-sEV, Control vs. sEV vs. Klotho-sEV) were then analyzed by hierarchical clustering, showing distinguishable (Fig. 5C-F).
Klotho-sEV treatment induced changes of proteins in AKI kidneys. Treatment with Klotho-sEV and sEV induced changes in proteins in AKI kidneys. (A) Identification of renal proteins by four-dimensional data-independent acquisition (4D-DIA) proteomics. (B) Histogram summarizing the differential expression proteins (DEPs) (fold change, Fc > 1.5 or Fc < 0.6667, and P value < 0.05) in the kidneys 3 d after hypertonic glycerol treatment. (C–F) Heatmaps showing the clustered DEPs from the comparisons of Control and Vehicle (C), Vehicle and Klotho-sEV (D), Vehicle, sEV, and Klotho-sEV (E), Control, Vehicle, and Klotho-sEV (F); the colors from blue to red indicate the expression of DEPs from low to high. (G–H) KEGG analysis from comparisons of Control and Vehicle (G) and Control, Vehicle, and Klotho-sEV (H)
We used the KEGG database to analyze DEPs in the vehicle vs. control comparison group, and the results showed that these DEPs were mainly enriched in metabolism, and metabolic pathways (295 proteins) were the most abundant. This suggests that metabolism plays a vital role in AKI. In particular, AKI had a strong impact on the PI3K-AKT, Rap1, mTOR, and PPAR signaling pathways (Fig. 5G). Further, KEGG enrichment analysis (Control vs. Vehicle vs. Klotho-sEV) showed that DEPs were mainly enriched in human diseases, organismal systems, and environmental information processing. Pathway enrichment revealed that the metabolic pathways, PI3K-AKT, Rap1, MAPK, mTOR, FoxO, and Ras pathways could participate in the disease process and repair of AKI (Fig. 5H).
Klotho-sEV treatment activated mTOR and MEK1/2 signal pathways in injured kidneys
Animal and clinical observational studies have shown that the Klotho protein not only serves as a biomarker of AKI but also functions as a promising reno-protective candidate [26, 37]. Western blotting was performed to further explore the effects of Klotho-sEV. The results showed that hypertonic glycerol treatment sharply down-regulated endogenous Klotho expression, whereas Klotho-sEV treatment reversed the levels of Klotho (Fig. 6A and B). Klotho is an essential component of endocrine fibroblast growth factor (FGF) receptor complexes as it is required for the high-affinity binding of FGF (19, 21, 23) to cognate FGF receptors [38]. mTOR and ERK1/2 are phosphorylated in the presence of Klotho and FGF [39, 40]. To validate the data from 4D-DIA proteomics and further determine the factors involved in the effects of Klotho-sEV on rhabdomyolysis-induced AKI, western blotting was performed. The pAKT levels were up-regulated after hypertonic glycerol administration (Fig. 6C). Surprisingly, we found that the phosphorylation levels of mTOR were up-regulated after Klotho-sEV treatment compared to those in the control, vehicle, and sEV groups (Fig. 6D). MEK1/2, which is upstream of ERK1/2, is involved in the MAPK pathway [41]. We found that pMEK1/2/MEK1/2 levels were dramatically down-regulated after hypertonic glycerol treatment, whereas Klotho-sEV treatment up-regulated the pMEK1/2/MEK1/2 levels (Fig. 6E).
mTOR and MEK1/2 phosphorylation were activated by Klotho-sEV in damaged kidneys. (A) GAPDH, Klotho, AKT, pAKT, pmTOR, MEK1/2, pMEK1/2 protein levels were determined using western blot. (B–E) Statistical quantification showed the relative expression of endogenous Klotho (B), pAKT (C), pmTOR (D), and pMEK1/2/MEK1/2 (E) in each group. Western blots indicated protein expression of injured kidneys in each group, 3 d after intramuscular injection of hypertonic glycerol. In all plots, mean with SD are shown. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by one-way ANOVA
Taken together, these data showed that Klotho-sEV restored Klotho expression in renal tissues and that the reno-protective effects of Klotho-sEV might be mediated by the phosphorylation of mTOR and MEK1/2.
sEV biodistribution and injury tropism
To elucidate the mechanisms by which sEV influence kidney function, in-vitro and in-vivo experiments were performed. Firstly, the uptake of sEV by HK2 cells was evaluated. mCherry-labelled sEV were successfully prepared (Supplementary Fig. 3A and B), and the uptake effects of HK2 cells on sEV were evaluated using flow cytometry. Using mCherry+ cell frequencies and MFI, we observed that that sEV were significantly taken up by HK2 cells after 48 h and 72 h of incubation, and that HK2 cells took up sEV in a time-dependent manner (Fig. 7A and B). Furthermore, we confirmed the in-vitro uptake effects of HK2 cells on sEV (red) using fluorescent microscopy (Fig. 7C). These results indicate that HK2 cells took in sEV in a time-dependent manner.
Preferential tropism of sEV in damaged kidneys. (A) Representative flow cytometric data of human renal proximal tubule (HK2) cells incubated with mCherry-labelled sEV. The levels of mCherry positive HK2 cells were shown. (B) The mean fluorescence intensity (MFI) of mCherry. (C) Detection of mCherry in HK2 cells with mCherry-labelled sEV incubation for 24 h by means of fluorescent microscopy. Original magnification ×200. (D) Ex vivo imaging of the main organs from healthy mice or mice suffered from rhabdomyolysis-induced AKI. (E) Statistical quantification of sEV distribution in healthy mice or mice suffering from rhabdomyolysis-induced AKI. In all plots, mean with SD are shown. **p < 0.01, ***p < 0.001, ****p < 0.0001 by unpaired t-test
Next, an endogenous labelling of sEV with a luciferase protein (NanoLuc-sEV) (Supplementary Fig. 3C-E) were used to elucidate the in-vivo distribution and tropism of sEV. Mice were injected intravenously with NanoLuc-sEV, then ex vivo imaging of the main organs was performed to determine the complete biodistribution of sEV. This analysis showed the organic distribution of sEV observed in the brains, lungs, hearts, livers, spleens, and kidneys, 1 h post-administration (Fig. 7D). For the rhabdomyolysis-induced AKI model, we observed significantly enhanced sEV accumulation in the kidneys, brains, and hearts, with most aggregation in the injured kidneys (Fig. 7D and E). Overall, these results indicate sEV preferential tropism to the injured kidneys in the setting of rhabdomyolysis-induced AKI.
Klotho-sEV and sEV contained abundant proteins
We next used iTRAQ to elucidate the protein components of Klotho-sEV and sEV. A total of 4347 proteins were identified in the two types of sEV, of which 40.9% (1776 proteins) were found in the VesiclePedia database (Fig. 8A). Conventional EV markers, CD63, CD81 and TSG101, were identified in both sEV (Supplementary Fig. 4A). Our data demonstrated that the abundance of 322 proteins (q value < 0.05) was significantly altered in the Klotho-sEV and sEV. Specifically, 212 proteins showed higher expression (Fc > 1.2), and 110 proteins showed lower expression (Fc < 0.8333) in Klotho-sEV than in sEV (Fig. 8B). The iTRAQ-based proteomic analysis identified 322 proteins that were categorized as cell components, the majority of which were assigned to the nucleus (114 proteins), cytosol (75 proteins), extracellular space (49 proteins), plasma membrane (32 proteins), and mitochondria (31 proteins) (Fig. 8C). Proteins with altered abundance levels were visualized in a volcano plot, which showed that most proteins were up-regulated in the Klotho-sEV (Fig. 8D). CC126, PHKG2, RMD1, LIPA3, LRP8, TPRX1, TBCA, Klotho, MYO9B, and RIPK2 were the top ten up-regulated proteins, whereas QCR1, ACSA, DKK1, PDK2, PKHA5, PEBP1, T2EA, CHID1, PTPRJ, and KCC2G were the top ten down-regulated proteins. The up-regulated expression of the Klotho protein in Klotho-sEV was confirmed. In addition, LRP8 (Fc 2.46), ARF5 (Fc 1.98), and FRAS1 (Fc 1.66) were also up-regulated (Fig. 8D and Supplementary Fig. 4B). It has been reported that LRP8, a low-density lipoprotein receptor-related protein 8, exerts anti-inflammatory activity by switching macrophages from the M1 phenotype to the M2 phenotype [42, 43]. ARF5 is a novel activator of mTOR signaling [44]; FRAS1 not only initiates kidney development but is also required for the formation of normal glomeruli [45, 46]. These data suggest that the expression of Klotho could change the protein components of sEV and that the abundant proteins in sEV might participate in reno-protection and tissue repair after injury in addition to Klotho.
sEV and Klotho-sEV were enriched in proteins. (A) Venn diagram of total proteins identified in sEV and Klotho-sEV compared to the VesiclePedia database. (B) Histogram summarizing differential expression proteins (DEPs) in sEV and Klotho-sEV (Fc > 1.2, Fc < 0.8333, and q value < 0.05). (C) Subcellular localization of DEPs. (D) Volcano plot of the proteins identified in sEV and Klotho-sEV. (E–G) KOG (E), GO (F), and KEGG pathway (G) analyses
We used the KOG database to analyze DEPs in the Klotho-sEV vs. sEV comparison group, and the results showed that these DEPs were mainly enriched in cellular processes and signaling, information storage and processing, and metabolism, while others were poorly characterized. Among cellular processes and signaling, signal transduction mechanisms, and post-translational modifications, protein turnover, and chaperones were the two main enriched categories. The main categories of information storage and processing were transcription, RNA processing, and modification (Fig. 8E). Furthermore, DEPs were searched in the GO database to determine enriched functional categories. According to the GO enrichment results, the DEPs of the Klotho-sEV vs. sEV comparison group were enriched in three main functional categories: biological processes, cellular components, and molecular functions. These three main functional categories were subdivided into 29, 19, and 13 smaller functional categories, respectively. Among the biological processes, cellular processes, metabolic processes, biological regulation, and regulation of biological processes were the four main enriched categories. Cells, cell parts, organelles, organelle parts, membranes, membrane-enclosed lumens, and macromolecular complexes were functional categories of DEPs that were widely enriched in the cellular components. The aggregation of DEPs in terms of molecular function was relatively concentrated primarily in terms of binding and catalytic activity (Fig. 8F). Finally, KEGG pathway analysis was performed. KEGG enrichment analysis showed that many co-differentially expressed proteins were enriched in cellular processes, environmental information processing, genetic information processing, human diseases, metabolism, and organismal systems. Cellular processes, transport and catabolism, cell growth and death, eukaryotic cellular communities, and cell mobility were the main categories. Signal transduction, signaling molecules and interactions, and membrane transport are the main categories of environmental information processing. Regarding genetic information processing, DEPs were mainly enriched in translation, folding, sorting and degradation, transcription and replication, and repair. The human disease category showed that these DEPs were mainly enriched in cancers, infectious diseases, endocrine and metabolic diseases, and neurodegenerative diseases. In addition, KEGG analysis revealed that, for metabolism, the DEPs were mainly enriched in carbohydrates, amino acids, and lipids, whereas for the organismal system, the DEPs were mainly enriched in the immune, endocrine, and nervous systems (Fig. 8G).
Overall, iTRAQ proteomics reports suggest that overexpression of Klotho by lentivirus infection could change the protein components of sEV, and other protein components in sEV might have reno-therapeutic effects in addition to Klotho.
Klotho-sEV and sEV carried abundant MiRNAs
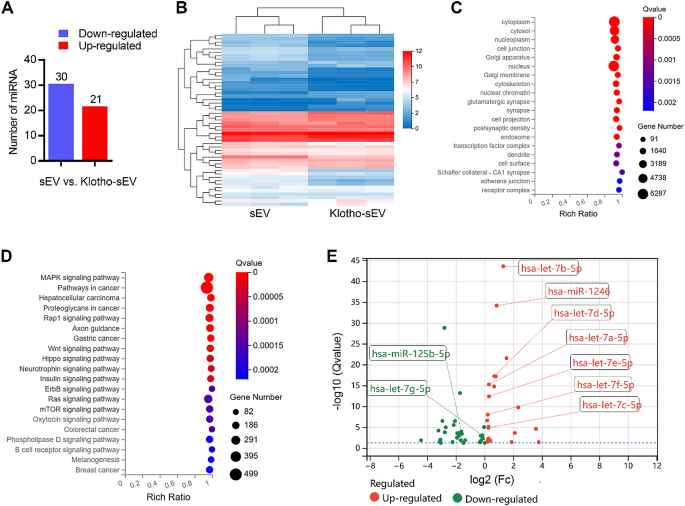
To identify miRNA components in Klotho-sEV and sEV, we performed small RNA sequencing. A total of 1007 miRNAs were identified in Klotho-sEV and sEV, and 9 of top 10 miRNAs were common, including hsa-let-7a-5p, hsa-miR-1246, hsa-miR-146a-5p, novel-hsa-miR264-3p, hsa-let-7f-5p, hsa-let-7i-5p, hsa-miR-127-3p, hsa-miR-100-5p, and hsa-miR-181a-5p. The abundance of 51 miRNA (|log2Fc| ≥ 0, q value ≤ 0.05) was obviously changed in Klotho-sEV and sEV. In addition, 30 miRNAs were down-regulated in Klotho-sEV than in sEV, whereas 21 miRNAs were up-regulated in Klotho-sEV compared to sEV (Fig. 9A). Hierarchical clustering showed that the miRNAs were distinguishable between Klotho-sEV and sEV (Fig. 9B). Target prediction of differentially expressed miRNAs using GO cellular component analysis showed that most miRNAs were assigned to the cytoplasm, nucleus, cytosol, and nucleoplasm (Fig. 9C). Target prediction by KEGG pathway analysis revealed that the altered miRNAs were involved in the MAPK signaling pathway, pathways in cancer, hepatocellular carcinoma, proteoglycans in cancer, and the Rap1 signaling pathway (Fig. 9D). The miRNAs with altered abundance levels were visualized in a volcano plot, which revealed that most miRNAs were down-regulated in Klotho-sEV (Fig. 9E). hsa-miR-141-3p, hsa-miR-375-3p, novel-hsa-miR245-5p, novel-hsa-miR62-5p, hsa-miR-3651, hsa-miR-182-5p, hsa-let-7b-5p, hsa-miR-1246, novel-hsa-miR289-5p, hsa-let-7d-5p were the top 10 up-regulated miRNA, while novel-hsa-miR30-3p, novel-hsa-miR148-3p, hsa-miR-130b-5p, hsa-miR-7704, hsa-miR-487a-5p, novel-hsa-miR293-5p, hsa-miR-10a-5p, novel-hsa-miR294-3p, hsa-miR-337-5p, hsa-miR-369-3p were the top 10 down-regulated miRNAs. The let-7 family and miR-1246 exert anti-inflammatory effects [47, 48]. We found that the let-7 family (hsa-let-7a-i), miR-1246 (Fc 1.76), and miR-125b-5p (Fc 0.33) were identified in both sEV groups (Fig. 9E and Supplementary Fig. 4D), suggesting that sEV containing miRNAs might play a role in reno-protection.
Abundant miRNAs were identified in sEV and Klotho-sEV. (A) Differentially expressed miRNAs were shown in the histogram (|log2Fc| ≥ 0, q value ≤ 0.05). (B) Heat map obtained by hierarchical cluster analysis. (C) Target prediction of differentially expressed miRNAs using GO cellular component analysis. (D) Target prediction of the differentially expressed miRNAs using KEGG pathway analysis. (E) Volcano plot of the miRNAs identified in sEV and Klotho-sEV
Biosafety of Klotho-sEV
We further investigated the biocompatibility of Klotho-encapsulated sEV in mice (Fig. 10A). The body weights of the mice receiving different treatments were recorded every other day for two weeks. Fluctuations in the body weight of mice in each experimental group tended to be stable (Fig. 10B). In addition, there was no significant difference in blood creatinine and urea levels between the different experimental groups (Fig. 1C and D). Histological evaluation showed no evidence of detectable toxicity in the brains, hearts, lungs, livers, spleens, and kidneys (Fig. 10E). Overall, these results revealed that both sEV and Klotho-sEV were almost harmless to healthy mice.
In-vivo biosafety of sEV and Klotho-sEV. (A) Schematic for the biosafety of Klotho-sEV/sEV in mice. (B) Body weight of mice in different experimental groups during the treatment cycle. (C & D) The levels of creatinine (C) and urea (D) in the serum of mice treated with different agents. (E) Representative images of HE staining analyses to the histopathological status of the brains, hearts, lungs, livers, spleens, and kidneys of mice treated with different agents. Original magnification ×200. In all plots, mean with SD are shown. (ns, no significant, p > 0.05 by one-way ANOVA)