Synthesis and charaterization of IMP@CM-PEP20 NPs
The synthetic scheme of IMP@CM-PEP20 NPs is illustrated in Fig. 1. As depicted in Fig. 2A, the IMP core exhibited a spherical morphology, while the CM-PEP20 appeared as a round membrane-like structure. The IMP@CM-PEP20 NPs displayed a distinct core–shell structure, demonstrating the successful coating of the IMP core with CM-PEP20. Furthermore, laser scanning confocal microscopy (CLSM) images revealed the complete colocalization of DiI (red)- labeled CM-PEP20 with DiO (green)-labeled PLGA core (Fig. 2B), further validating the successful CM-PEP20 coating. Elemental mapping of IMP NPs showeda uniform distribution of Mn, C, and O elements (Fig. 2C), confirming the effective loading of MnO₂ onto the PLGA core. Additionally, X-ray photoelectron spectroscopy spectra were performed to analyze the valence state of Mn ions within IMP NPs. The Mn2p spectrum confirmed the presence of Mn2+, Mn3+ and Mn4+ in IMP, with Mn4⁺ being the predominant species (Fig. 2D), which endows IMP NPs with a potent GSH-depleting capacity. Inductively coupled plasma optical emission spectrometry (ICP-OES) determined the Mn ion content in IMP NPs to be 5.1%. The hydrodynamic diameter and zeta potential of the IMP core and IMP@CM-PEP20 were 196 ± 19.8 nm, 11.25 ± 0.56 mV, and 220.7 ± 23.8 nm, 21.3 ± 0.52 mV, respectively (Fig. S1, Supporting Information). The negative surface charge of IMP@CM-PEP20 NPs contributed to their good dispersion stability. The drug loading efficiency and encapsulation efficiency of IR780 in IMP NPs was 9.3% and 46.5%, respectively. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was utilized to evaluate the retention of membrane proteins during PEP20 ligation and coating. As shown in Fig. 2E, both the protein profiles of CM-PEP20 and PLGA@CM-PEP20 closely matched that of the purified CM, indicating negligible protein loss. To determine the optimal conjugation ratio, different weight ratios of PEP20: CM (based on protein content) were investigated and analyzed using flow cytometry (FCM). PEP20 and CM were labelled by FITC and PE/cy7 fluorescent dyes, respectively. After synthesis and purification, the resulting PE/cy7-CM-PEP20-FITC was incubated with 4 T1 cells for 8 h. Following PBS washing to remove the non-phagocytosed PE/cy7-CM-PEP20-FITC, the ratio of PE/cy7-CM and PEP20-FITC was quantified by FCM (Fig. 2F). The results revealed a mass-dependent increase in binding efficiency, which exceeded 90%. However, no further enhancement was observed when the PEP20:CM ratio reached or surpassed 1:2 (Fig. 2G, H). Hence, a PEP20:CM ratio of 1:2 was selected as optimal. Collectively, these results confirm the successful fabrication of IMP@CM-PEP20 NPs.
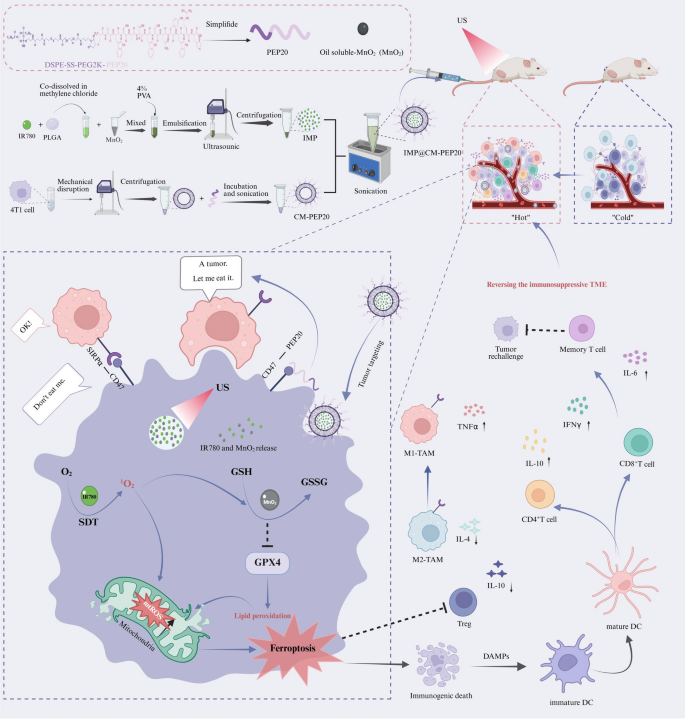
Scheme illustration of IMP@CM-PEP20 NPs and their antitumor mechanisms. IMP@CM-PEP20 NPs were synthesized by a series of processes including membrane extraction, a single emulsion method and sonication. Due to the homotypic and active targeting of CM and PEPE20, IMP@CM-PEP20 nanoparticles efficiently accumulate at the tumor site. Under US irradiation, IR780 converts oxygen (O2) into singlet oxygen (1O2). The generated 1O2 and MnO2 oxidize GSH into GSSG, downregulating GPX4 expression, facilitating LPOs accumulation, ultimately inducing tumor ferroptosis. The generated reactive oxide species (ROS) promotes the repolarization of M2-TAMs into M1-TAMs. Meanwhile, PEP20 specifically binds to CD47 on the tumor membrane, promoting the phagocytosis of tumor cells by M1-TAMs. Additionally, the resultant ferroptosis triggers tumor ICD, which shifts the “cold” TME into the “hot” one by promoting DC maturation, priming T cell immunity, and finally establishing a long-term memory immune response. TME: tumor microenvironment; SDT: sonodynamic therapy; DAMPs:damage associated molecule patterns;TAM: tumor-associated macrophages; DC: dendritic cells; Tregs: regulatory T cells
Characterization of IMP@CM-PEP20 NPs. A Representative TEM images and size distribution of IMP nanoparticles, CM-PEP20, and IMP@CM-PEP20 NPs. B Representative CLSM images of colocalization of IMP@CM-PEP20 NPs. DiO: green. DiI: red. Scale bar: 10 μm. C Representative element mapping images of IMP nanoparticle. Scale bar: 100 μm. D XPS survey spectra of IMP nanoparticles. E SDS–PAGE analysis of proteins extracted from PLGA nanoparticles, fresh CMs, PLGA@CM, PLGA@CM-PEP20 NPs. F Schematic illustration of investigation of the binding ratio of PEP20 and CM via FCM. G Typical FCM plots of PEP20 and cell membrane under different mass ratios and H their corresponding quantitative analysis (n = 3). I TEM images of IMP@CM-PEP20 NPs under different conditions. J Release curves of Mn ions from IMP@CM-PEP20 NPs under different conditions. K Schematic illustration of the GSH-consumption and SDT effect of IMP NPs. L Assessment of GSH consumption after treated with IMP nanoparticles. Time-dependent (a) or concentration-dependent (b) GSH consumption by incubating with IMP nanoparticles. M) Assessment of Singlet oxygen-generation after treated with IMP or IP nanoparticles under US irradiation. a Time-dependent production of singlet oxygen induced by IMP nanoparticles under US irradiation b Singlet oxygen production induced by IP nanoparticles (2 mg/mL) or different concentration of IMP nanoparticles under US irradiation
To evaluate the colloidal stability of IMP@CM-PEP20 NPs, the NPs were dispersed in different media, such as deionized water (ddH₂O), RPMI-1640 medium (with or without 10 mM GSH), and incubated for 24 h. Their hydrodynamic sizes at different time points were recorded and showed no significant changes, indicating good colloidal stability (Fig S2, Supporting Information). Transmission electron microscopy (TEM) images further confirmed the structural integrity of IMP@CM-PEP20 NPs under both GSH-free and GSH-containing conditions, showing uniform morphology without notable differences. However, under the condition of 10 mM GSH combined with US irradiation, the NPs exhibited internal shrinkage, aggregation, and partial degradation, likely due to US-induced cavitation effects (Fig. 2I).
The release of Mn ion from IMP@CM-PEP20 NPs was also evaluated using ICP-OES (Fig. 2J). As we expected, in the presence of GSH, Mn ion release was significantly enhanced in the presence of GSH, compared to GSH-free conditions. Moreover, US irradiation further accelerated Mn ion release, presumably due to ultrasound-induced structural disruption of the NPs.
To evaluate the GSH-depleting capacity of IMP, 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) was used as a UV probe via the Ellman’s assay [29]. The reaction equations are as follows:
$$Mn^{4 + } + GSH\to _{{}} Mn^{2 + } + GSSG$$
(1)
$$2GSH + DTNB\to _{{}} GSSG + TNB$$
(2)
After incubation with IMP@CM-PEP20 NPs, GSH levels decreased in a time- and concentration-dependent manner (Fig. 2L). To assess the reactive oxygen species (ROS) generation capacity of IMP NPs, Singlet Oxygen Sensor Green (SOSG) was used as a fluorescence probe. As shown in Fig. 2M, under US irradiation, the fluorescence intensity (FI) of SOSG increased progressively with time and concentration, indicating the strong ROS-generating capability of IMP. Additionally, the relative FI (FI/F0) of SOSG in IMP group (2 mg/mL) at a rate comparable to that observed with IR780@PLGA (IP) NPs (2 mg/mL). These findings demonstrated the efficient GSH depletion and ROS generation capacities of IMP@CM-PEP20 NPs.
Biosafety, cytotoxicity and cellular uptake of IMP@CM-PEP20 NPs
Encouraged by the favorable physicochemical performance of IMP@CM-PEP20 NPs, their antitumor performance in vitro was further investigated. The biosafety of the NPs was first evaluated in 4 T1 tumor cells and human umbilical vein endothelial cells (HUVECs) at varying concentrations using a standard Cell Counting Kit-8 assay (Fig. 3A). At 500 μg/mL, minimal cytotoxicity was observed in HUVECs, whereas 4 T1 cell viability decreased to approximately 70%. This differential cytotoxicity may be attributed to the elevated GSH levels in the TME of 4 T1 cells, which promotes GSH depletion by MnO₂ and subsequently induces ferroptosis. At 1000 μg/mL, both cell types exhibited significant reductions in viability. Therefore, 500 μg/mL was selected as the optimal concentration for subsequent in vitro experiments.
Study of anti-tumor performance and cellular uptake in vitro. A Viability of 4 T1 and HUVEC cells after incubating with different concentration of IMP@CM-PEP20 nanoparticles for 24 h (n = 3). B Viability of 4 T1 cells after different treatments (n = 3). D Fluorescence images of 4 T1 cells stained with Calcein-AM (live) and PI (dead) after different treatments and C its quantitative analysis (n = 3). Scale bar: 200 μm. Representative CLSM images of cellular uptake behaviors of DiO-labled PLGA, DiO-labled PLGA@CM and DiO-labled PLGA@CM-PEP20 nanoparticles in E 4 T1 cells and F macrophages, and G–H their corresponding FCM analysis (n = 3). DiO: green. DAPI: blue. Scale bar: 10 μm. Statistical significance was performed by one-way ANOVA with a Tukey post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Next, the cytotoxicity of various treatments was assessed in 4 T1 cells, including PBS (control), IR780@PLGA@CM (IP@CM), IR780/MnO₂@PLGA (IMP), IR780/MnO₂@PLGA@CM (IMP@CM), PEP20, ferrostatin-1 (FER-1) + IMP@CM-PEP20, and IMP@CM-PEP20, with or without US irradiation (Fig. 3B). Among all groups, the IMP@CM-PEP20 + US group exhibited the lowest cell viability, indicating its potent tumoricidal activity. No significant cytotoxicity was observed in either the PBS or PEP20-treated groups, suggesting that PEP20 alone does not adversely affect 4 T1 cell viability. Interestingly, the IMP@CM + US group showed higher cell viability than the IMP@CM-PEP20 + US group, which may be attributed to the specific binding affinity of PEP20 to CD47 on tumor cells. This binding likely facilitated enhanced nanoparticle accumulation at the tumor cell surface, thereby improving the targeted delivery and therapeutic efficacy of IMP@CM-PEP20 NPs. To further validate the role of ferroptosis in cell death, 4 T1 cells were pre-incubated with FER-1, a ferroptosis inhibitor known to block LPO accumulation [26], for 40 min prior to nanoparticle treatment. Cells treated with FER-1 + IMP@CM-PEP20 + US exhibited significantly higher viability than those treated with IMP@CM-PEP20 + US alone, further supporting that ferroptosis was effectively induced in the latter group.
These findings were corroborated by live/dead staining using calcein acetoxymethyl ester (Calcein-AM) and propidium iodide (PI) (Fig. 3C, D). Among all treatment groups, the IMP@CM-PEP20 + US group displayed the most intense red fluorescence (PI) and the weakest green fluorescence (Calcein-AM), visually confirming its pronounced cytotoxic effect and potential for anti-tumor therapy.
To further evaluate the tumor targeting capacity of the NPs, DiO-labeled PLGA NPs were coated with either CM or CM-PEP20, and their cellular uptake was compared in 4 T1 cells. CLSM images (Fig. 3E) revealed that DiO-labeled PLGA@CM NPs exhibited greater internalization than bare DiO-labeled PLGA NPs, due to the homotypic affinity of CM. Notably, DiO-labeled PLGA@CM-PEP20 NPs showed significantly enhanced cell uptake than other NPs, suggesting that PEP20 decoration further enhances tumor-targeting efficiency. Quantitative analysis by FCM corroborated these findings, confirming increased endocytosis of DiO-labeled PLGA@CM-PEP20 NPs in 4 T1 cells (Fig. 3G). To investigate immune evasion, macrophages were incubated with the same DiO-labeled nanoparticle formulations. Both CLSM and FCM results (Fig. 3F, H) demonstrated significantly reduced uptake of DiO-labeled PLGA@CM-PEP20 NPs by macrophages compared to bare DiO-labeled PLGA NPs. Interestingly, a modest increase in internalization was observed in macrophages treated with PLGA@CM-PEP20 NPs compared to PLGA@CM NPs. This may be attributed to the incorporation of a small fraction of PEP20 onto the CD47-expressing CM during synthesis, partially blocking the “don’t eat me” signal and thereby promoting macrophage-mediated uptake.
Study of ferroptosis induced performance in vitro
It is well-established that ROS generated during SDT consume intracellular GSH, leading to GPX4 inactivation and ultimately inducing irreversible ferroptosis [14, 30, 31]. To evaluate intracellular ROS levels, we employed 2′,7′-dichlorofluorescin diacetate (DCFH-DA) staining. As both IR780 and US irradiation are essential for ROS generation, groups lacking either component exhibited negligible ROS production. Compared to the IMP@CM + US group, the IP@CM + US group showed reduced ROS levels due to the absence of MnO2 and its GSH-depleting effect. Similarly, the IMP + US group displayed lower intracellular ROS levels relative to the IMP@CM + US group, likely due to the lack of homotypic targeting conferred by the CM coating. Notably, the IMP@CM-PEP20 + US group generated the highest ROS levels among all groups, indicating that PEP20 enhances active targeting and nanoparticle uptake, thereby promoting ROS accumulation. Furthermore, pre-incubation with FER-1 significantly reduced ROS production in the FER-1 + IMP@CM-PEP20 + US group, corroborating the involvement of ferroptosis. Among all the US-irradiated groups, the IMP@CM-PEP20 + US-treated group exhibited the most intense green fluorescence, supporting the hypothesis that MnO₂-mediated GSH depletion effectively reduced ROS scavenging and enhanced oxidative stress (Fig. 4A, D).
Study of ferroptosis induction. A Fluorescence images of ROS (green) level in 4 T1 cells after different treatments and D its quantitative analysis (n = 3). Scale bar: 100 μm. B Fluorescence images of C11-BODIPY581/591 dye–stained 4 T1 cells after different treatments and E its quantitative analysis (n = 3). Scale bar: 100 μm. C Fluorescence images of JC-1 dye–stained 4 T1 cells after different treatments and F its quantitative analysis of relative mitochondrial membrane potential (Δψm) (n = 3). Scale bar: 100 μm. G GSH level in 4 T1 cells after different treatments (n = 3). H GPX4 activity in 4 T1 cells after different treatments (n = 3). Statistical significance was performed by one-way ANOVA with a Tukey post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
We subsequently investigated the combined effects of SDT and MnO2 on GSH depleting and ferroptosis. Intracellular GSH levels in 4 T1 cells were quantified using a commercial GSH/GSSG assay kit. As shown in Fig. 4G, the PEP20-treated group showed no significant change in GSH levels compared to the control, indicating that PEP20 alone does not affect intracellular redox balance. In contrast, all other treatment groups exhibited significantly reduced GSH levels, with the most pronounced depletion observed in the IMP@CM-PEP20 + US group. Interestingly, the IP@CM + US-treated group also showed a notable decline in GSH levels relative to the control, confirming that ROS generated by SDT oxidize GSH to GSSG, consistent with previous reports [32]. Given that GSH depletion leads to GPX4 inactivation [33], we further assessed GPX4 activity using a commercial GPX4 activity assay kit. The results paralleled the GSH assay, with the lowest GPX4 activity observed in the IMP@CM-PEP20 + US group (Fig. 4H). Since inactivation of GPX4 disrupts the detoxification of LPOs, LPO accumulation serves as a hallmark of ferroptosis. To visualize LPO accumulation, we used the LPO-sensitive probe C11-BODIPY581/591, which localizes to cell membranes and undergoes a fluorescence shift from red (590 nm) to green (510 nm) upon oxidation [34]. The IMP@CM-PEP20 + US-treated group exhibited the weakest red fluorescence and the strongest green fluorescence, indicating the greatest LPO accumulation and, thus, the greatest ferroptotic response. In contrast, co-treatment with the FER-1 (FER-1 + IMP@CM-PEP20 + US group) significantly reduced green fluorescence, further confirming that the observed effects were ferroptosis-dependent (Fig. 4B, E).
A hallmark of ferroptosis is the disruption of mitochondrial structure and function, including a marked reduction in mitochondrial membrane potential (Δψm) [35]. To evaluate mitochondrial damage, we employed a JC-1 assay to assess Δψm. JC-1 is a fluorescent lipophilic carbonyl cyanine dye that accumulates in mitochondria in a potential-dependent manner. In healthy mitochondria with high Δψm, JC-1 aggregates in the mitochondrial matrix and emits strong red fluorescence (Ex = 585 nm, Em = 590 nm). In contrast, in depolarized or damaged mitochondria, JC-1 remains in its monomeric form, emitting green fluorescence (Ex = 514 nm, Em = 529 nm). As revealed in Fig. 4C and F, all groups treated with SDT and MnO2 exhibited a markedly reduce in Δψm, as evidenced by decreased red fluorescence and increased green fluorescence. Notably, the IMP@CM-PEP20 + US group displayed almost complete loss of red fluorescence and the strongest green fluorescence signal, indicating the most pronounced mitochondrial depolarization among all groups.
These findings suggest that the combination of SDT and GSH depletion synergistically enhances ferroptotic cell death, and that the IMP@CM-PEP20 + US treatment robustly induces ferroptosis through severe mitochondrial dysfunction in 4 T1 cells.
Anti-tumor immunity mechanism in vitro
Ferroptosis is a key mode of ICD, which shifts the tumor immune environment from a “cold”, immunosuppressive state into a “hot”, immune-responsive state [7, 8]. Inspired by the potent ferroptosis-inducing performance of the IMP@CM-PEP20 + US strategy, we next assessed its anti-tumor immune performance. The distinct traits of ICD are mediated by DAMPs, including ATP secretion, passive release of HMGB-1, and CRT exposure [8]. To assess these markers, we measured ATP levels using an ATP assay kit and evaluated CRT exposure and HMGB1 release via immunofluorescence staining. CLSM images of CRT revealed substantial translocation of CRT immunofluorescence staining. CLSM images of CRT revealed substantial translocation of CRT to the surface of 4 T1 cells in the IP@CM + US, IMP + US, IMP@CM + US, and IMP@CM-PEP20 + US-treated groups (Fig. 5A, C). Notably, the IMP@CM-PEP20 + US-treated group exhibited the most significant CRT exposure. In contrast, the FER-1 + IMP@CM-PEP20 + US group displayed reduced CRT exposure, suggesting that inhibiting ferroptosis in 4 T1 cells attenuates the ICD effect. Immunofluorescence staining of HMGB1 revealed similar results (Fig. 5B, D). As shown in Fig. 5E, the highest extracellular ATP levels were observed in the IMP@CM-PEP20 + US-treated group. This remarkable ICD-induced effect may be attributed to the tumor-targeting capabilities of CM and PEP20, ferroptosis induction by MnO2, and the synergistic effects of SDT.
Study of anti-tumor immunity in vitro. A Representative CLSM images of CRT exposure after different treatment and C its quantitative analysis (n = 3). Scale bar: 25 μm. B HMGB-1 passive release after different treatments and D its quantitative analysis (n = 3). Scale bar: 25 μm. E The release of ATP in 4 T1 cell culture medium after different treatments (n = 3). F Schematic illustration of in vitro investigation into IMP@CM-PEP20 NPs induced immunomodulation using bone marrow-derived macrophages (BMDMs) and -dendritic cells (BMDCs). G Schematic diagram depicting PEP20-mediated phagocytosis of 4 T1 cells by M1-BMDMs. H Representative FCM plots indicating BMDC maturation after different treatments and J their quantitative analysis (n = 3). I Representative FCM histograms indicating M1-BMDM repolarization after different treatments and K their corresponding M1/M2 ratios (n = 3). L Representative FCM histograms indicating the phagocytosis of 4 T1 cell by M1-like BMDMs and M their quantitative analysis (n = 3). Statistical significance was performed by one-way ANOVA with a Tukey post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
The TME is immunosuppressive, featured by insufficient cytotoxic T lymphocytes (CTLs), abundant pro-tumoral macrophages, rich anti-inflammatory cytokines, and large amounts of immunosuppressive cells [36]. ICD promotes the maturation of DCs, enhancing tumor antigen presentation, and triggering tumor-specific T cell immune responses, ultimately reversing the immunosuppressive TME [8]. To assess this, we studied DC maturation. Bone marrow-derived dendritic cells (BMDCs) were incubated with supernatants from 4 T1 cells treated with different interventions for 4 h, followed by analysis via FCM (Fig. 5F). The lipopolysaccharide (LPS)-treated group was used as a positive control. DCs which express both CD86 and CD80 proteins are defined as mature DCs. Compared with the control group, the PEP20-treated group didn’t significantly promote DC maturation. In contrast, all other treatment groups substantially enhanced DC maturation, with maturation rates at least seven times higher than the control. Notably, the maturation rate of DCs in the IMP@CM-PEP20 + US-treated group (54.1%) was nearly as high as that in the LPS-treated group (58.8%) (Fig. 5H, J), suggesting a significantly enhancement of antigen presentation effect and the potential to trigger robust T cell immune responses.
ROS have been well-documented to reprogram M2-like macrophages into the pro-inflammatory M1 phenotype [14, 37]. Given the potent ROS-generating capability of our designed nanoplatforms, we next investigated macrophage polarization using bone marrow-derived macrophages (BMDMs) treated with various interventions and analyzed the phenotypic changes via FCM (Fig. 5F). As revealed in Fig. 5I, G, and Fig. S3 from Supporting Information, higher CD86 expression and lower CD206 expression in BMDMs were observed in all US irradiation-involved groups, verifying effective M2-to-M1 macrophage repolarization. Among all SDT-involved groups, the IMP@CM-PEP20 + US-treated group demonstrated the highest M1 macrophage proportion, comparable to that of the lipopolysaccharide (LPS)-treated positive control. This enhanced polarization effect can be attributed to the active targeting conferred by PEP20 and the GSH-depleting capability of MnO₂. Based on these findings, we further examined whether PEP20 (a CD47 inhibitory peptide) could promote the phagocytosis of 4 T1 tumor cells by M1-like macrophages. BMDMs were first induced into M1-like macrophages using LPS. 4 T1 cells were pre-incubated with PBS, CM, CM-PEP20 for 4 h, followed by co-culture with M1-like BMDMs for 8 h. The phagocytosis efficiency was then quantified by FCM (Fig. 5G). As shown in Fig. 5L and M, CM-PEP20 significantly enhanced the phagocytosis of 4 T1 cells by M1-like BMDMs, whereas no significant difference was observed between the CM-treated and control groups. This result suggests that PEP20 can effectively block the “don’t eat me” signal mediated by CD47, thereby facilitating macrophage-mediated clearance of tumor cells.
In conclusion, our multifunctional nanoplatform not only induced robust tumor ICD and promoted dendritic cell maturation, but also effectively reprogrammed immunosuppressive M2-like macrophages into tumor-suppressive M1-like macrophages and enhanced their phagocytic activity. These findings underscore the promising potential of our strategy in reversing the immunosuppressive TME.
Biodistribution and antitumor performance in vivo based on the 4 T1 primary tumor model
In vivo targeting, and biodistribution
Building upon the excellent tumor-targeting capability of PLGA@CM-PEP20 NPs observed in vitro, the biodistribution of IR780@PLGA@CM-PEP20 (IP@CM-PEP20) NPs was further evaluated in vivo using 4 T1 tumor-bearing mice (Fig. 6A). The targeting efficacy was assessed by monitoring the FI of the near-infrared dye IR780. Mice were intravenously administered IP@CM-PEP20 NPs or their derivative formulations, and fluorescence imaging was performed at various time points using an in vivo imaging system. As shown in Fig. 6B and E both the IP@CM and IP@CM-PEP20 groups exhibited significantly stronger tumor-associated FI compared to the IP group, confirming the tumor-targeting contributions of the CM coating and PEP20 peptide. Notably, the FI in the IP@CM group peaked at 24 h post-injection and subsequently declined, whereas the IP@CM-PEP20 group maintained elevated FI levels even at 72 h. These results underscore the superior and prolonged tumor-targeting capability of the CM-PEP20 modification. To further evaluate tissue distribution, mice were sacrificed at 72 h post-injection for ex vivo fluorescence imaging of major organs, tumor tissues and blood samples. Tumors from the IP@CM-PEP20 group exhibited the highest FI among all tissues, even exceeding the FI detected in the liver, which is typically the primary site of nanoparticle accumulation. This observation strongly supports the efficient and selective tumor-targeting performance of the IP@CM-PEP20 formulation (Fig. 6C, F). Additionally, analysis of blood samples collected at 72 h revealed a significantly stronger IR780-associated FI in the IP@CM-PEP20 group (Fig. 6D, G), indicating that the biomimetic coating effectively prolonged systemic circulation. This extended circulation time likely facilitated enhanced accumulation of drug-loaded nanoparticles at the tumor site, thereby improving the overall delivery efficiency.
Study on in vivo biodistribution and biosafety performance. A Diagram illustrating the process of in vivo targeting assessment, including modeling, administration, and monitoring protocol. B In vivo fluorescence images of 4 T1 tumor-bearing mice at different time points after intravenous injection of IR780@PLGA, IR780@PLGA@CM, and IR780@PLGA@CM-PEP20 NPs and E their quantitative analysis of average radiant efficiency (n = 3). C ex vivo fluorescence imaging of the resected main organs, tumors at 72 h post-injection and F their quantitative analysis of average radiant efficiency (n = 3). D ex vivo fluorescence imaging of the collected blood samples at 72 h post-injection and G their quantitative analysis of average radiant efficiency (n = 3). H Diagram illustrating assessment of in vivo biosafety of IMP@CM-PEP20 NPs. I Hemolysis ratio of water, 0.9% Nacl and different concentrations of IMP@CM-PEP20 NPs and J their quantitative analysis (n = 3). K Heat map of biochemical blood test results in healthy mice treated for 5 courses (n = 3). Statistical significance was performed by one-way ANOVA with a Tukey post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
In vivo systemic biosafety investigation
To investigate systemic biosafety, healthy mice were intravenously injected with IMP@CM-PEP20 NPs every two days until day 9. On day 9, the mice were sacrificed, and blood samples were collected to evaluate liver and kidney function. including alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), uric acid (UA), and creatinine (CRE), remained within normal physiological ranges (Fig. 6K). In addition, a hemolysis assay was also conducted to further examine the hemocompatibility of the NPs. As shown in Fig. 6I, J, negligible hemolysis (< 2%) was observed even at an IMP concentration as high as 2 mg/mL, further confirming the excellent blood compatibility and overall biosafety of the IMP@CM-PEP20 formulation.
In vivo anticancer performance in 4 T1 primary tumor-bearing mice
Inspired by the impressive in vitro anti-tumor performance of IMP@CM-PEP20 NPs, we further investigated their anti-tumor efficacy in vivo using 4 T1 tumor-bearing mice. Following the therapeutic schedule outlined in Fig. 7A, 4 T1 tumor-bearing mice received intravenous injections every two days with the following groups: normal saline (control), IP@CM + US, IMP, IMP + US, IMP@CM + US, PEP20, FER-1 + IMP@CM-PEP20 + US, and IMP@CM-PEP20 + US. Tumor volume and body weight were monitored throughout the treatment period. While the IMP-only group showed no significant tumor growth inhibition compared to the control, all other treatment groups exhibited varying degrees of tumor growth suppression. Notably, the IMP@CM-PEP20 + US group demonstrated the most pronounced therapeutic effect, with the lowest final tumor volume (as low as 1.858 mm3), and one mouse in this group achieved complete tumor regression (Fig. 7B–D). To investigate the histopathological changes underlying this therapeutic effect, tumors were excised after five treatment cycles and subjected to hematoxylin and eosin (H&E) and Ki-67 staining. H&E staining revealed extensive tissue damage characterized by enlarged intracellular spaces and nuclear condensation, indicative of severe tumor cell apoptosis or ferroptosis (Fig. S4). Ki-67 immunohistochemistry confirmed minimal tumor cell proliferation in the IMP@CM-PEP20 + US group, aligning with the H&E results (Fig. S5).
Study on antitumor performance in primary tumor mouse model. A Scheme of treatment regimen for assessing the antitumor efficiency in 4 T1 primary tumor model. B Photographic images of resected tumors on day 20 after receiving different treatments (n = 4). C Average tumor growth curves and D individual primary tumor growth curves after different treatments (n = 4). E Body weight curves of mice in each group during the whole process of treatments (n = 4). F Representative images DHE staining in cryo-sectioned tumors after different treatments and H their quantitative analysis (n = 3). Scale bar: 50 μm. G Representative images of GPX4 immunofluorescence staining of resected tumors after different treatments and I) their quantitative analysis (n = 3). Scale bar: 100 μm. J Western blotting analysis of the expression level of GPX4 in 4 T1 primary tumor tissues after different treatments. Statistical significance was performed by one-way ANOVA with a Tukey post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Importantly, no significant changes in body weight were observed in any group, and H&E staining of major organs (heart, liver, spleen, lung, kidney) showed no pathological abnormalities, affirming the systemic biosafety of all treatments (Fig. 7E; Fig. S6, Supporting Information).
Study of ferroptosis-induced performance in vivo
Given the robust ferroptosis-inducing capability of IMP@CM-PEP20 + US in vitro, we further evaluated its efficacy in vivo. ROS production in tumor tissues was assessed using dihydroethidium (DHE) staining on cryo-sectioned samples. As depicted in Fig. 7F and H, minimal green fluorescence was observed in the control and PEP20 groups, indicating negligible ROS levels. In contrast, all other groups exhibited increased DHE fluorescence, with the IMP@CM-PEP20 + US group showing the strongest signal. This result confirms that our strategy achieves effective ROS generation in vivo, consistent with the in vitro DCFH-DA results. To assess ferroptosis more directly, GPX4 expression levels were examined via immunofluorescence staining. Compared to the control group, GPX4 FI was reduced in all treatment groups except for the PEP20 group, suggesting varying levels of ferroptosis induction. The lowest GPX4 signal was observed in the IMP@CM-PEP20 + US group, while co-treatment with the ferroptosis inhibitor FER-1 partially restored GPX4 expression, supporting that ferroptosis was indeed induced by our combined treatment strategy (Fig. 7G, I). These findings were further validated by western blot analysis of GPX4 expression (Fig. 7J; Fig. S7, Supporting Information). Collectively, these results indicate that the IMP@CM-PEP20 + US strategy efficiently induces ferroptosis in vivo through the synergistic effects of SDT, GSH depletion by MnO₂, and enhanced tumor targeting.