Formulation and characterization of GCS-IO NPs
The morphology of the GCS-IO NPs was observed using transmission electron microscopy (TEM) (Fig. 2A). Compared to uncoated IO (left), GCS-IO NPs (right) exhibited a more uniform and stable dispersion. TEM images confirmed that the IO NPs were encapsulated within the GCS matrix, forming a spherical and well-distributed structure with minimal aggregation. The GCS coating was crucial for stabilizing the particles and preventing clumping, ensuring better colloidal stability in aqueous environments [43]. The dynamic light scattering (DLS) analysis (Fig. 2B) indicated that the mean hydrodynamic diameter of GCS-IO NPs was approximately below 500 nm, a significant increase compared to bare IO NPs, highlighting the effective coating by GCS. The particle size distribution was narrow, suggesting a monodispersed system ideal for in vivo applications. Zeta potential measurements (Fig. 2C) showed that GCS-IO NPs had a higher positive charge compared to bare IO NPs, indicating enhanced colloidal stability due to surface modification with GCS, which should improve cellular interactions and prolong circulation time in the bloodstream.
Formulation and characterization of glycol chitosan iron oxide nanoparticles (GCS-IO NPs). (A) Transmission electron microscopic (TEM) images showing the morphology of uncoated IO NPs (left) and GCS-IO NPs (right). GCS-IO NPs displayed a more-uniform, spherical structure with minimal aggregation, confirming successful encapsulation of IO NPs within the GCS matrix. (B) Particle size distribution of GCS-IO NPs measured using dynamic light scattering (DLS). GCS-IO NPs showed a mean hydrodynamic diameter of approximately below 500 nm. (C) Zeta potential measurements comparing IO NPs and GCS-IO NPs, indicating a significant increase in surface charge for GCS-IO NPs due to the GCS coating, contributing to improved stability. (D) Fourier-transform infrared (FTIR) spectra of GCS, IO NPs, and GCS-IO NPs, confirming the presence of Fe-O bonds and the successful coating of GCS around IO NPs. (E) X-ray diffraction (XRD) patterns of GCS, IO NPs, and GCS-IO NPs, demonstrating the crystalline structure of IO in GCS-IO NPs. (F) Magnetic properties of IO NPs and GCS-IO NPs measured using a superconducting quantum interference device (SQUID) magnetometer. GCS-IO NPs exhibited superparamagnetic behavior with negligible remanence and coercivity, making them suitable for magnetic targeting applications. (G) Photothermal performance of PBS, IO NPs, and GCS-IO NPs under 808-nm near-infrared (NIR) laser irradiation (2.45 W/cm²). GCS-IO NPs showed a significant temperature rise, confirming their photothermal conversion capability. Infrared thermal images of PBS, IO NPs, and GCS-IO NPs during NIR irradiation, illustrating the localized heating effect of GCS-IO NPs, reaching temperatures of over 50 °C, highlighting their potential for photothermal therapy (PTT)
The Fourier-transform infrared (FTIR) spectra of GCS-IO NPs, GCS, and IO NPs (Fig. 2D) confirmed the successful encapsulation of IO NPs within the GCS matrix. Characteristic peaks at 3200–3500 cm⁻¹ corresponding to O-H and N-H stretching vibrations [44] in GCS-IO NPs were observed, confirming the presence of hydroxyl and amine groups from GCS. Peaks at around 600 cm⁻¹, attributed to Fe-O bonds, were clearly visible in IO NPs and GCS-IO NPs, further verifying the presence of IO within the NP system. The x-ray diffraction (XRD) analysis (Fig. 2E) revealed distinct diffraction peaks at 30°, 35°, 43°, and 57°, which corresponded to the crystalline structure of IO NPs [45]. A broadening of peaks in GCS-IO NPs indicated that the GCS coating introduced some amorphous character, yet the overall crystalline structure of the IO core remained intact, supporting its use for magnetic applications. Magnetic characterization using a superconducting quantum interference device (SQUID) magnetometer (Fig. 2F) demonstrated that GCS-IO NPs exhibited superparamagnetic behavior, with a near-zero remanence and coercivity. This is a desirable trait for biomedical applications such as magnetic resonance imaging (MRI) and targeted drug delivery, as the NPs can be efficiently guided using an external magnetic field without retaining residual magnetization once the field is removed. The reduced magnetic moment compared to bare IO NPs was attributed to the GCS coating, which slightly hindered the magnetic response, but not to a degree that would impair its utility in magnetic-targeted applications. The photothermal conversion efficiency of GCS-IO NPs was evaluated under 808-nm near-infrared (NIR) laser irradiation (2.45 W/cm²) in an aqueous dispersion (Fig. 1G). Compared to phosphate-buffered saline (PBS) and IO NPs, GCS-IO NPs exhibited a significantly higher temperature increase, reaching over 50 °C after 10 min of irradiation. This superior photothermal performance can be attributed to the combination of IO’s intrinsic photothermal properties [46] and enhanced colloidal stability provided by the GCS coating. The data suggested that GCS-IO NPs can be highly effective for photothermal therapy (PTT) applications, allowing for localized hyperthermia in tumor tissues with minimal damage to surrounding healthy tissues. Infrared thermal imaging (Fig. 1G) further confirmed the photothermal effect, showing a stark contrast in temperature changes between the PBS control and GCS-IO NPs group. Images highlighted the localized heating effect of GCS-IO NPs upon laser irradiation, making them promising candidates for remote non-invasive cancer therapies that leverage hyperthermia.
Results presented in Fig. 1 collectively demonstrate the successful formulation and characterization of GCS-IO NPs, which combine the magnetic properties of IO with the biocompatibility and stability provided by the GCS coating. The NPs exhibited an excellent photothermal conversion efficiency, magnetic responsiveness, and stable dispersion, making them well-suited for multifunctional theranostic applications, including MRI, PTT, and targeted drug delivery.
Formulation and characterization of CHL-GCS-IO NPs
The dihydroethidium (DHE) fluorescence intensity data (Fig. 3A) revealed the ability of CHL (107 cells/mL) plus various amounts of GCS-IO NPs to produce reactive oxygen upon light irradiation, evaluated for various GCS-IO NPs feeding amounts (0, 5, 50, 125, 250, and 500 µg). The DHE assay was used to detect oxygen generation [47] under 660-nm laser irradiation for 0, 5, and 10 min. The formulated CHL plus GCS-IO NPs exhibited a time- and concentration-dependent increase in oxygen generation, as indicated by the significant rise in the DHE fluorescence intensity at higher NP feeding amounts (250 and 500 µg) and an extended exposure time (10 min). The data suggested that this oxygen could efficiently modulate oxygen levels within the TME, which is critical for overcoming hypoxic conditions typically found in solid tumors [48]. Specifically, 10 min of irradiation at a 125-µg feed amount resulted in a nearly 25% increase in the fluorescence intensity, indicating robust oxygen production compared to the other groups, all of which showed minimal oxygen generation. Considering the highest efficacy, the notable enhancement in oxygenation supported selecting CHL-GCS-IO NPs at a concentration of 125 µg for subsequent experiments. This dosage proved to be an effective therapeutic platform for alleviating tumor hypoxia and enhancing the efficacy of dynamic therapies, such as PST. Figure 3B further supports these findings with fluorescence microscopic images. The difference in the fluorescence intensity before and after 10 min of 660-nm light irradiation demonstrated that the formulated CHL-GCS-IO NPs exhibited significantly higher oxygen production compared to untreated CHL. The pronounced increase in DHE fluorescence (blue signal) after 10 min of exposure indicated effective ROS generation in the presence of NPs. The morphological uniformity of the fluorescence distribution also highlighted the efficient interaction between light irradiation and CHL-GCS-IO NPs, resulting in sustained oxygen release. These results suggested the potential of this system to overcome hypoxia-driven chemoresistance by modulating the TME. A zeta potential analysis (Fig. 3C) was performed to assess the colloidal stability [49] of CHL, GCS-IO NPs, and CHL-GCS-IO NPs. Bare CHL exhibited a negative zeta potential of approximately -20 mV, typical for microalgae due to the presence of negatively charged functional groups on their surface. GCS-IO NPs showed a positive zeta potential (ca. +20 mV), attributed to the cationic nature of GCS used for coating the IO NPs. Upon conjugation of the GCS-IO NPs to CHL cells, the zeta potential of CHL-GCS-IO NPs shifted slightly to around + 10 mV, suggesting GCS-IO NPs attachment and charge neutralization between the anionic CHL surface and cationic GCS-IO NPs. The positively charged surface of CHL-GCS-IO NPs enhanced their stability, improving cellular interactions and reducing aggregation, which is crucial for in vivo applications, especially for prolonging circulation times and effective tumor targeting. The zeta potential measurements of our developed formulation at different time points (0 days, 1 day, and 7 days) in PBS. The results show that the zeta potential remains consistently above + 10 mV across all time points, indicating good stability of the CHL-GCS-IO NPs in PBS over time (Figure S1A). Fluorescence microscopic data (Fig. 3D) indicated the conjugation of GCS-IO NPs (with green FITC fluorescence) onto CHL (red chlorophyll fluorescence). In CHL-GCS-IO NPs samples, a distinct overlay of FITC (green) and chlorophyll (red) fluorescence was observed, confirming the formation of hybrid CHL-GCS-IO NPs. Merged images revealed a homogeneous distribution of the GCS-IO NPs on the CHL surface, signifying efficient conjugation between GCS-IO NPs and CHL cells. Fluorescent signals from both FITC and chlorophyll remained distinct and stable after conjugation, demonstrating that the conjugation process did not adversely affect the inherent properties of either the GCS-IO NPs or CHL. These results suggest that CHL-GCS-IO NPs retained photosynthetic capabilities of CHL while integrating magnetic and photothermal properties of GCS-IO NPs, creating a multifunctional system for theranostic applications. Figure 3E compares chlorophyll activities of different formulations, showing the fluorescence intensity of chlorophyll [50] in CHL, CHL-GCS-IO NPs, GCS-IO NPs, CHL-DMSO, and CHL-HEAT under various conditions. CHL-GCS-IO NPs exhibited slightly increased chlorophyll activity compared to untreated CHL alone, which is expected due to the positive attenuation caused by NP binding. However, the fluorescence intensity of CHL-GCS-IO NPs remained significantly higher than those of the CHL-DMSO and CHL-HEAT groups, indicating that chlorophyll activity was preserved despite conjugation with GCS-IO NPs. The CHL sample exposed to 50 °C retained a fluorescence intensity of about 1 unit with an emission wavelength between 650 and 700 nm, indicating stable optical properties under thermal conditions.
Characterization and performance of Chlorella glycol chitosan iron oxide nanoparticles (CHL-GCS-IO NPs). (A) DHE fluorescence intensity data demonstrating oxygen generation by CHL (107 cells/mL) formulated with various concentrations of GCS-IO NPs (0, 5, 50, 125, 250, and 500 µg) under 660-nm laser irradiation for 0, 5, and 10 min. Data show a time- and concentration-dependent increase in oxygen production, with high reactive oxygen species (ROS) generation tendency at higher NP concentrations and longer irradiation times. (B) Fluorescence microscopic images showing DHE fluorescence in CHL and CHL-GCS-IO NPs before and after 10 min of 660-nm laser irradiation. CHL-GCS-IO NPs exhibited higher fluorescence intensities, indicating enhanced oxygen generation. Scale bar: 10 μm. (C) Zeta potential measurements for CHL, GCS-IO NPs, and CHL-GCS-IO NPs. The shift in the zeta potential from − 20 mV for CHL to + 10 mV for CHL-GCS-IO NPs suggests successful conjugation of GCS-IO NPs and improved colloidal stability. (D) Fluorescence microscopy showing conjugation of GCS-IO NPs (green FITC fluorescence) onto CHL (red chlorophyll fluorescence). The merged image confirms successful conjugation, with distinct fluorescent signals for FITC and chlorophyll, indicating that the photosynthetic and magnetic properties were retained. Scale bar: 50 μm. (E) Comparison of chlorophyll activities across various formulations (CHL, CHL-GCS-IO NPs, GCS-IO NPs, CHL-DMSO, and CHL-HEAT). CHL-GCS-IO NPs demonstrated slightly increased chlorophyll activity compared to untreated CHL. (F) RDPP data of CHL and CHL-GCS-IO NPs evaluated at 0 min and 10 min light irradiation
This preserved chlorophyll activity is critical for the functionality of CHL as a photosynthetic agent, enabling it to continuously produce oxygen under light irradiation, which is essential for enhancing PST therapeutic effects in tumor environments. In Fig. 3F, the photobleaching behavior and fluorescence stability of CHL and CHL-GCS-IO NPs were assessed using the RDPP assay to evaluate oxygen production at 0 and 10 min of light irradiation. At 0 min, both CHL and CHL-GCS-IO NPs displayed strong fluorescence signals, indicating high initial fluorescence intensity. However, after 10 min of irradiation, CHL exhibited a faint red fluorescence, while CHL-GCS-IO NPs showed less detectable fluorescence. This suggests that CHL underwent partial photobleaching, retaining residual fluorescence, whereas CHL-GCS-IO NPs facilitated complete fluorescence quenching. The observed difference in fluorescence retention may be attributed to the interaction between CHL and the nanoparticle matrix, which likely enhances photobleaching or promotes signal quenching. These results provide insights into the oxygen-generating capacity of CHL-GCS-IO NPs in comparison to free CHL.
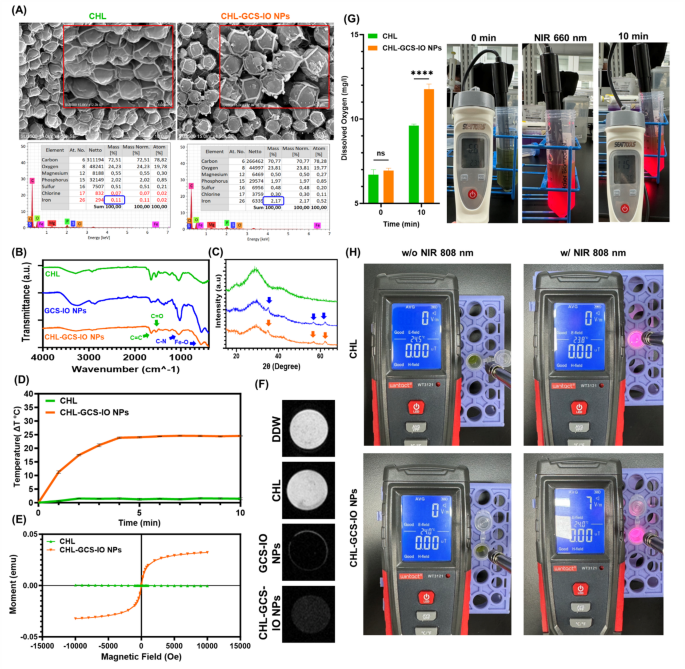
SEM images and elemental composition data from energy-dispersive X-ray spectroscopy (EDS) provided insights into structural differences between CHL and CHL-GCS-IO NPs (Fig. 4A). SEM images revealed clear distinctions in morphology, with CHL-GCS-IO NPs showing surface roughness and enhanced structural definition compared to the smoother surface of CHL alone. The iron (Fe) content significantly increased in CHL-GCS-IO NPs, confirming the successful conjugation of IO NPs to Chlorella cells, with the Fe mass increasing from 0.11% in CHL to 2.17% in CHL-GCS-IO NPs. This substantial rise in the Fe content is key to the enhanced magnetic properties of the system, enabling magnetic guidance and potential for theranostic applications. Additionally, other elemental components, such as oxygen and sulfur, remained relatively constant, indicating that the core structure of Chlorella was preserved during the NP attachment process.
Characterization of Chlorella glycol chitosan iron oxide nanoparticles (CHL-GCS-IO NPs) (A) Scanning electron microscopic (SEM)-energy-dispersive x-ray spectroscopic (EDS) analysis. SEM images comparing surface morphologies of CHL and CHL-GCS-IO NPs. The SEM image of CHL shows a smoother surface, while CHL-GCS-IO NPs exhibit surface roughness, indicative of successful conjugation with GCS-IO NPs. Elemental analysis by EDS demonstrates a significant increase in iron (Fe) content in CHL-GCS-IO NPs (2.17%) compared to CHL (0.11%), confirming NP attachment. (B) Fourier-transform infrared (FTIR) spectroscopy: FTIR spectra of CHL, GCS-IO NPs, and CHL-GCS-IO NPs. The spectrum of CHL-GCS-IO NPs shows characteristic peaks of C = O stretching (~ 1700 cm⁻¹), C-N stretching (~ 1400 cm⁻¹), and Fe-O (~ 600 cm⁻¹), confirming successful conjugation and preservation of bioactivity. (C) X-ray diffraction (XRD) analysis: XRD patterns of CHL, GCS-IO NPs, and CHL-GCS-IO NPs. Crystalline peaks corresponding to Fe₃O₄ (iron oxide) were observed in CHL-GCS-IO NPs, confirming the incorporation of magnetic NPs into the Chlorella system. (D) Photothermal heating effect (photothermal therapy (PTT)): Temperature elevation profiles of CHL and CHL-GCS-IO NPs under 808-nm NIR irradiation. CHL-GCS-IO NPs exhibited a substantial temperature increase of over 20 °C after 10 min, highlighting their potential for PTT, whereas CHL alone showed a minimal temperature change. (E) Superconducting quantum interference device (SQUID) magnetization curves: SQUID analysis of CHL and CHL-GCS-IO NPs. The magnetization curve for CHL-GCS-IO NPs indicates superparamagnetic behavior with significantly higher saturation magnetization (Ms), essential for magnetic guidance and in vivo therapeutic applications. (F) T2-weighted magnetic resonance imaging (MRI) analysis: T2-weighted MRI images of double-distilled water (DDW), CHL, GCS-IO NPs, and CHL-GCS-IO NPs. CHL-GCS-IO NPs demonstrated strong T2 contrast, enhancing their potential as MRI contrast agents for precise tumor imaging in theranostic applications. (G) Photosynthetic oxygen production under light irradiation. The graph compares the oxygen production rates of Chlorella (CHL) and Chlorella-conjugated glycol iron oxide nanoparticles (CHL-GCS-IO NPs) under identical light exposure conditions. CHL-GCS-IO NPs exhibit significantly enhanced oxygen production compared to CHL, indicating improved photosynthetic efficiency due to the presence of glycol iron oxide nanoparticles. (H) Electromagnetic wave responses of Chlorella (CHL) and CHL-GCS-IO NPs under light irradiation. The electromagnetic field (E-field) measurements demonstrate a negligible change in CHL samples under light exposure, while CHL-GCS-IO NPs exhibit a substantial increase in electromagnetic activity, likely attributed to the conductive and electromagnetic-responsive properties of the glycol iron oxide nanoparticles
FTIR spectroscopy (Fig. 4B) was used to identify functional groups present in GCS-IO NPs, CHL, and CHL-GCS-IO NPs. For GCS-IO NPs, characteristic absorption bands were observed for GCS and IO, including peaks corresponding to Fe-O and C-N bonds. In CHL-GCS-IO NPs, the C = O stretching peak (~ 1700 cm⁻¹) [51] and the C-N stretching at ~ 1000 cm⁻¹ [52] were indicative of successful conjugation between the NPs and the Chlorella surface. The presence of the Fe-O absorption band (at ~ 600 cm⁻¹) [53] confirmed the attachment of IO NPs. The overlap of these peaks suggested that the GCS-IO NPs did not interfere with the key functional groups of CHL, preserving the system’s bioactivity while enhancing its functional properties.
The XRD analysis (Fig. 4C) revealed the crystalline structure of CHL-GCS-IO NPs compared to CHL and GCS-IO NPs. Distinct peaks corresponding to crystalline phases of IO were observed in both GCS-IO NPs and CHL-GCS-IO NPs, confirming the presence of magnetite/maghemite NPs. Diffraction peaks at around 2θ = 30° and 60° well matched with characteristic peaks of Fe₃O₄, further validating the successful incorporation of magnetic NPs into the CHL system. Notably, CHL alone displayed a relatively amorphous pattern, while CHL-GCS-IO NPs showed additional crystalline peaks due to IO, indicating that the NPs contributed to the overall crystalline structure without disrupting CHL’s natural morphology.
The photothermal heating effect of CHL-GCS-IO NPs was examined under 808-nm NIR irradiation (Fig. 4D). CHL-GCS-IO NPs exhibited a significant temperature change increase compared to CHL alone, with temperatures rising by over 20 °C after 10 min of irradiation. This demonstrated the potential of CHL-GCS-IO NPs to effectively generate heat, supporting their use in PTT applications. In contrast, the temperature of CHL alone remained nearly constant, indicating that CHL exhibited no intrinsic photothermal properties without the attached IO NPs. The rapid and substantial temperature rise in the CHL-GCS-IO NPs group was crucial for inducing hyperthermia in tumor tissues, which can contribute to the destruction of cancer cells.
A SQUID analysis (Fig. 4E) was performed to evaluate the magnetic properties of CHL-GCS-IO NPs compared to CHL alone. The magnetization curve for CHL-GCS-IO NPs showed clear superparamagnetic behavior, with a significant increase in the magnetic moment compared to the non-magnetic CHL control. The saturation magnetization (Ms) of CHL-GCS-IO NPs was much higher, further confirming the successful incorporation of IO NPs [54]. This magnetic responsiveness is critical for the guidance and actuation of CHL-GCS-IO NPs in vivo under external magnetic fields, facilitating targeted delivery to tumor sites and enhancing therapeutic outcomes.
T2-weighted MRI results (Fig. 4F) demonstrated the potential of CHL-GCS-IO NPs as contrast agents. While CHL and double-distilled water (DDW) showed minimal contrast in MRI images, GCS-IO NPs and CHL-GCS-IO NPs produced strong T2 contrast, as indicated by the darker signal in MRI scans. The CHL-GCS-IO NPs group exhibited superior T2-weighted contrast compared to GCS-IO NPs alone, likely due to the enhanced particle dispersion and stability provided by attachment to CHL. These results suggested that CHL-GCS-IO NPs hold promise as MRI contrast agents for non-invasive tumor imaging, enabling precise localization of tumors in addition to their therapeutic functions. CHL-GCS-IO NPs characterization indicated their multifunctional properties, combining the photosynthetic activity of CHL with the magnetic and photothermal properties of GCS-IO NPs. These attributes make CHL-GCS-IO NPs an ideal platform for multimodal theranostic applications, capable of MRI-guided therapy, photothermal ablation, and immune activation through photosynthetic oxygen generation. The robust magnetic properties, high photothermal conversion efficiency, and excellent MRI contrast further support their use in dynamic cancer therapies, targeting solid tumors in hypoxic microenvironments.
Figure 4G and H present the results of photosynthetic oxygen evolution and the electromagnetic wave responses under light irradiation for Chlorella (CHL) cells and Chlorella-conjugated glycol iron oxide nanoparticles (CHL-GCS-IO NPs). The photosynthetic oxygen production data in Fig. 4G demonstrate a significant enhancement in oxygen evolution upon light irradiation for both CHL and CHL-GCS-IO NPs, with CHL-GCS-IO NPs showing a markedly higher output. This suggests that the conjugation with glycol iron oxide nanoparticles enhances the efficiency of photosynthesis, likely due to the improved light-harvesting capability of the nanoparticle-functionalized cells. The CHL at 25 °C and 50 °C showed photosynthetic oxygen production even at elevated temperature (Figure S1B). Previous studies have indicated that microalgae may exhibit some level of tolerance to both thermal stress and free radicals [55,56,57]. It is important to highlight that the 808 nm light used for photothermal therapy (PTT) is applied in the final stage of the treatment. Prior to this, CHL-GCS-IO NPs are exposed to 660 nm light, which is responsible for driving the photosynthetic processes. In the final step, the CHL-GCS-PPy NPs undergo exposure to 808 nm light for the PTT treatment. While this step may potentially cause the death of the microalgae, any surviving CHL-GCS-PPy NPs would be eliminated by the body’s immune system and subsequently excreted.
In Fig. 4H, the electromagnetic wave data reveal distinct responses between the CHL and CHL-GCS-IO NPs samples. The electromagnetic field (E-field) measurements under light irradiation show negligible changes for pure CHL, while CHL-GCS-IO NPs exhibit a noticeable increase in electromagnetic activity. This enhanced response in CHL-GCS-IO NPs can be likelyattributed to the conductive properties of the glycol iron oxide nanoparticles, which facilitate the generation and transmission of electromagnetic signals under illumination.
These findings highlight the dual functionality of CHL-GCS-IO NPs, combining enhanced photosynthetic activity with electromagnetic responsiveness. The integration of nanoparticles with Chlorella cells not only amplifies biological photosynthesis but also introduces a potential for applications in biohybrid systems, such as light-driven electromagnetic devices or bioenergy systems.
In vitro biochemical assays
The cell viability assay presented in Fig. 5A highlights the effects of CHL and CHL-GCS-IO NPs on MB49 bladder cancer cells in the absence of NIR light irradiation. Without NIR activation, CHL and CHL-GCS-IO NPs moderately reduce MB49 cell viability at concentrations of up to 100 × 104 cells/mL, with both treatment groups maintaining over 60% viability across all concentrations. This indicates that without PTT or PST activation, CHL and CHL-GCS-IO NPs exhibited moderate cytotoxicity in the absence of light stimulation. Importantly, CHL-GCS-IO NPs showed slightly lower cell viability at the high concentration (100 × 104 cells /mL), but the difference was not statistically significant (ns), further confirming that NP conjugation did not inherently induce cytotoxicity under non-irradiative conditions.
Evaluation of MB49 cell viability and reactive oxygen species (ROS) generation following treatment with Chlorella (CHL) and CHL-glycol chitosan iron oxide nanoparticles (CHL-GCS-IO NPs) under near infrared (NIR) irradiation. (A) Cell viability of MB49 cells treated with CHL and CHL-GCS-IO NPs at various concentrations (0–500 µg/mL) without NIR irradiation. Both CHL and CHL-GCS-IO NPs exhibited moderate cytotoxicity, with cell viability remaining above 60% (0–100 µg/mL). Statistical significance: ns, not significant, **** p < 0.0001. (B) Cell viability of MB49 cells treated with CHL and CHL-GCS-IO NPs with or without NIR irradiation (660 + 808 nm). CHL-GCS-IO NPs, when irradiated with 660- and 808 nm-light, resulted in a significant decrease in cell viability (~ 70% cell death). In contrast, CHL alone produced a minimal reduction in viability. (C) ROS generation by MB49 cells, as determined by a DCFH-DA assay. Fluorescence microscopic images (top: without NIR; bottom: with 660-nm NIR) and corresponding quantitative analysis (bottom graph) demonstrating significant ROS production induced by CHL and CHL-GCS-IO NPs under NIR irradiation. The CHL-GCS-IO NPs group exhibited significantly higher ROS generation than CHL alone, and the addition of H₂O₂ further amplified ROS production. Scale bar: 100 μm. (D) Cellular Prussian blue staining. Scale bar: 50 μm
In contrast, Fig. 5B demonstrates the combined effects of CHL, CHL-GCS-IO NPs, and NIR irradiation (660 and 808 nm) on MB49 cell viability. Being subjected to NIR irradiation, cell viability was significantly reduced by CHL-GCS-IO NPs in a wavelength-dependent manner. Under 660-nm irradiation, CHL-GCS-IO NPs caused a substantial decrease in cell viability (approximately 50% cell death), whereas 808-nm irradiation enhanced this effect, leading to an even more-pronounced reduction in viability (~ 65% cell death). The combination of both 660- and 808-nm wavelengths resulted in the highest level of cell death (~ 70%), underscoring the synergistic effects of PTT and PST mediated by CHL-GCS-IO NPs. In comparison, CHL alone also induced some reduction in cell viability under NIR irradiation, but its effects were significantly less pronounced than CHL-GCS-IO NPs. This data confirmed the superior phototherapeutic efficacy of CHL-GCS-IO NPs, particularly when both wavelengths were employed, likely due to enhanced oxygen generation and heat production facilitated by IO NPs.
Results of in vitro qualitative and quantitative analyses (Fig. 5C) indicated cellular ROS generation using the DCFH-DA assay under NIR 660-nm irradiation. Fluorescence images and intensity quantification showed a clear difference in ROS production across the various treatment groups. In the absence of NIR irradiation, all groups, including the control, CHL, and CHL-GCS-IO NPs groups, exhibited minimal ROS generation, as indicated by the weak green fluorescence. However, upon 660-nm NIR irradiation, both CHL and CHL-GCS-IO NPs induced substantial ROS generation, with CHL-GCS-IO NPs exhibiting a significantly stronger fluorescence signal (p < 0.0001), indicating higher ROS levels compared to CHL alone. The inclusion of H2O2 in the CHL-GCS-IO NPs group further amplified ROS production, demonstrating the ability of the formulation to catalyze H2O2 into oxygen and enhance the oxygen-generating effect (p < 0.0001). Quantitative data, as measured by ImageJ software, confirmed these findings, with CHL-GCS-IO NPs producing the highest fluorescence intensity, reinforcing their capability for potent ROS-mediated cancer cell destruction through PST.
Figure 5D shows that CHL-GCS-IO NPs significantly induce iron accumulation in MB49 cells, as evidenced by Prussian blue staining, compared to control and CHL-only treatments. This effective delivery of iron oxide nanoparticles through Chlorella likely initiates ferroptosis, a form of programmed cell death linked to lipid peroxidation. The reduction in MB49 cell viability in the CHL-GCS-IO NP group supports this therapeutic effect. Consequently, these bioengineered nanoparticles demonstrate a dual function, combining Chlorella’s biocompatibility with the cytotoxic potential of iron-induced ferroptosis, presenting a promising cancer therapy strategy.
Together, these results underscore the dual PTT and PST capabilities of CHL-GCS-IO NPs, making them a highly effective theranostic platform for bladder cancer therapy, particularly in overcoming hypoxia-driven resistance within the TME. Their ability to generate substantial ROS and induce significant cell death under NIR irradiation highlights their potential for dynamic cancer treatment applications. The combination of IO NPs and CHL enhanced both magnetic responsiveness and photosynthetic oxygen production, offering a multifaceted approach to tumor therapy that integrates PTT, PST, and immune-activating strategies.
Fluorescence microscopic results in Fig. 6A highlight polarization of RAW264.7 macrophages from the M0 state to the M1 state, marked by cluster of differentiation 86 (CD86; M1) expression, upon treatment with CHL and CHL-GCS-IO NPs. Without NIR irradiation, the lipopolysaccharide (LPS)-treated group showed high CD86 expression, indicating efficient polarization into M1 macrophages. In contrast, treatment with CHL, and CHL-GCS-IO NPs produced minimal CD86 expression under these conditions, suggesting a limited ability to induce M1 polarization in the absence of external stimulation. The GCS-IO NPs produced less CD86 expression with and without NIR irradiation. Upon 660-nm NIR irradiation, CHL-GCS-IO NPs produced significantly increased CD86 expression, indicating enhanced M1 polarization compared to CHL alone. This effect highlights the potential of CHL-GCS-IO NPs to modulate macrophage polarization, driving proinflammatory (M1) activation under light stimulation. Qualitative data from microscopic images were supported by a quantitative analysis using ImageJ software, showing a significant increase in fluorescence intensities for the CHL-GCS-IO NPs group after NIR irradiation, further confirming the role of NIR-activated CHL-GCS-IO NPs in promoting M1 polarization. The ability to modulate macrophage activation could play a crucial role in reshaping the tumor immune microenvironment, making it more conducive to immune-mediated cancer cell clearance.
Polarization of RAW264.7 macrophages induced by Chlorella (CHL) and CHL-glycol chitosan iron oxide nanoparticles (GCS-IO NPs) with and without near infrared (NIR) irradiation. (A) M0 to M1 polarization (CD86 expression). Fluorescence microscopic images of RAW264.7 macrophages showing CD86 (M1 marker, red) and nuclei stained with DAPI (blue) under various treatments: control, lipopolysaccharide (LPS), CHL, GCS-IO and CHL-GCS-IO NPs, with and without 660-nm NIR irradiation. LPS treatment showed significant CD86 expression, indicative of M1 polarization. The 660-nm NIR irradiation of CHL-GCS-IO NPs significantly enhanced M1 polarization, as indicated by an increased CD86 fluorescence intensity compared to CHL treatment alone. Quantitative analysis of the fluorescence intensity, using ImageJ software, confirmed the significant increase in CD86 expression by CHL-GCS-IO NPs under NIR irradiation. (B) M2 to M1 re-polarization (CD206 to CD86 expression shift). Fluorescence microscopic images showing CD206 (M2 marker, green) and CD86 (M1 marker, red) expressions by RAW264.7 macrophages treated with IL-4 (an M2 polarization inducer), followed by CHL and CHL-GCS-IO NPs, with and without 660-nm NIR irradiation. Without NIR, IL-4 maintained high CD206 and low CD86 expression, indicating strong M2 polarization. With NIR irradiation, CHL-GCS-IO NPs induced a significant shift, reducing CD206 and enhancing CD86 expression, indicating re-polarization from M2 to M1. Quantitative analysis using ImageJ software demonstrated a significant increase in CD86 and a decrease in CD206 expression after NIR irradiation in the CHL-GCS-IO NP group (**** p < 0.0001). Scale bar: 200 μm
Figure 6B presents data on the re-polarization of M2 macrophages (induced by interleukin (IL)-4) back to the M1 state using CHL and CHL-GCS-IO NPs, marked by both CD206 (M2) and CD86 (M1) expressions. Without NIR irradiation, IL-4-treated macrophages displayed high CD206 (M2) expression, indicative of successful M2 polarization. The addition of CHL or CHL-GCS-IO NPs had partial effects on reducing M2 polarization under non-irradiated conditions. The GCS-IO NPs produced no CD206 expression with and without NIR irradiation. However, with NIR irradiation, CHL-GCS-IO NPs caused significantly increased CD86 expression (p < 0.0001), while simultaneously reducing CD206 expression, indicating an effective re-polarization from the anti-inflammatory M2 phenotype (mimicking the TME) back to the proinflammatory M1 state. CHL treatment alone also demonstrated some ability to re-polarize macrophages from M2 to M1, although to a lesser extent than by CHL-GCS-IO NPs. These results suggested that light-triggered activity of CHL-GCS-IO NPs can effectively switch macrophages from an immunosuppressive to a proinflammatory phenotype, which is critical for enhancing antitumor immune responses. The quantitative analysis using ImageJ software supported these findings, showing a significant increase in M1 markers and a decrease in M2 markers after NIR irradiation in the CHL-GCS-IO NPs group (p < 0.0001). This re-polarization ability represents a promising strategy for overcoming tumor-associated macrophage (TAM)-mediated immunosuppression in the TME. The immunofluorescence data suggested that immune cells, such as macrophages, can be educated, trained, and reprogrammed in response to PST with CHL-GCS-IO NPs upon 660-nm irradiation. Together, data from Fig. 6 underscore the dual capability of CHL-GCS-IO NPs to drive macrophage polarization toward a tumor-suppressive M1 phenotype, both from naïve macrophages and re-polarized M2 macrophages. The NIR-triggered modulation of macrophage activity suggests a novel immunotherapeutic approach that could be integrated with other cancer treatment modalities to enhance the immune response within the TME, potentially leading to improved therapeutic outcomes.
The blood test analysis (Figure S1C) provides a comparative evaluation of blood cell populations over time (14 days) across different formulation-treated groups, with a focus on red blood cells (RBCs; reference range: approximately 7.8–10.6 M/µL), reticulocytes (reference range: approximately 200–500 K/µL), and white blood cells (WBCs; reference range: approximately 2–10 K/µL).
In the control group, the RBC count was 10.47 M/µL, reticulocyte count was 476.4 K/µL, and WBC count was 5.04 K/µL. By day 14, the CHL-GCS-IO NPs (mag.) group showed a slight recovery in RBCs to 8.67 M/µL, a mild reduction in reticulocytes to 512.5 K/µL, and a stable WBC count at 6.34 K/µL.
These results suggest that treatment with CHL-GCS-IO NPs does not significantly affect hematopoiesis and maintains blood cell levels within biosafe reference ranges over time. Distinct blood cell types are represented by specific colors in the scatter plots—RBCs (red), reticulocytes (purple), and WBCs (cyan)—which highlight the distribution and clustering patterns of these populations across both control and treated groups.
In vivo examinations
Encouraged by the promising in vitro results, we further expanded our investigation by conducting in vivo studies to rigorously evaluate the performance of our design in animal models, aiming to validate our key hypothesis. Figure 7A outlines the timeline for the animal experiments, detailing the sequence of MB49 tumor cell injection, formulations administration, NIR irradiation, and tumor size monitoring. The treatment period spanned from day 0 (injection of CHL, GCS-IO NPs, or CHL-GCS-IO NPs) to day 14 (sacrifice and final tumor size measurement). The biodistribution analysis presented in Fig. 7B evaluated the accumulation and clearance of CHL and CHL-GCS-IO NPs in major organs and tumors in MB49 tumor-bearing mice using in vivo imaging system (IVIS) imaging. In and 7B after an IV injection, CHL-GCS-IO NPs (with magnetic targeting) demonstrated the highest accumulation in the tumor site compared to the CHL and CHL-GCS-IO NPs groups without magnetic guidance. The fluorescence intensity in the tumor was significantly higher in the CHL-GCS-IO NPs group under magnetic guidance, indicating effective magnetic targeting and enhanced tumor retention of CHL-GCS-IO NPs. Fluorescence was also detected in the liver and kidney, consistent with typical polymer formulations biodistribution. A quantitative analysis of the fluorescence intensity confirmed a significant increase in tumor accumulation by the magnetically targeted CHL-GCS-IO NPs group, while other groups showed relatively lower tumor targeting. In contrast, the clearance of NPs after 7 days showed relatively minimal fluorescence in most organs and the tumor. The CHL-GCS-IO NPs group with magnetic targeting retained a higher residual fluorescence signal in the tumor compared to the CHL and other groups, although the overall signal had decreased, indicating effective metabolism and clearance of NPs from the system. At the 14th day of IVIS imaging (Fig. S1D), the different formulation-treated groups—Control (+ Magnet), CHL (+ Magnet), CHL-GCS-IO NPs, and CHL-GCS-IO NPs + Magnet—show no visible accumulation in major organs, including the heart, spleen, liver, lungs, and kidneys. This suggests that the formulations may have been effectively cleared from circulation. Figure 7C illustrates thermal imaging results of tumor-bearing mice subjected to 808-nm NIR irradiation. Thermal images reveal the photothermal effects of the different NP treatments. The control and CHL groups showed minimal temperature increases (~ 32 °C), indicating the absence of significant photothermal activity. In contrast, GCS-IO NPs and CHL-GCS-IO NPs (with magnetic targeting) exhibited substantial temperature increases, with CHL-GCS-IO NPs reaching over 50 °C after 5 min of NIR irradiation, confirming their superior photothermal conversion capability. The maximum temperature observed in the CHL-GCS-IO NPs group (50.8 °C) demonstrated their high efficiency for heat generation, which is critical for PTT applications. The ability to achieve such high temperatures within a short period is indicative of the strong photothermal response of the CHL-GCS-IO NPs, enhanced by the combination of magnetic targeting and NIR activation. This thermal rise is essential for inducing localized hyperthermia, which contributes to destruction of tumor cells in vivo. Together, these results confirmed the multifunctionality of CHL-GCS-IO NPs for magnetically guided targeting, efficient tumor accumulation, and potent photothermal effects under NIR irradiation. This system holds promise for future applications in magnetic-targeted PTT and other multimodal cancer therapies, particularly in overcoming challenges posed by tumor hypoxia and improving the precision of therapeutic interventions.
(A) Schematic timeline of animal experiments, including MB49 tumor cell injection, formulations administration, near infrared (NIR) irradiation, and tumor size monitoring. This image was created and edited using BioRender software. (B) Biodistribution of Chlorella (CHL) and CHL-glycol chitosan iron oxide (GCS-IO) NPs in MB49 tumor-bearing mice 1 h after an intravenous injection, both with and without magnetic targeting. Fluorescence intensity maps show the accumulation of CHL-GCS-IO NPs in tumors and organs (heart, liver, spleen, lungs, and kidneys). A quantitative analysis indicated a significant increase in tumor accumulation by CHL-GCS-IO NPs in the magnetic-targeted group compared to the other groups. Biodistribution of CHL and CHL-GCS-IO NPs 7 days post-injection and treatments. Fluorescence imaging demonstrated clearance of the formulations, with a residual signal observed in the tumor of CHL-GCS-IO NPs of the magnetic-targeted group. A quantitative analysis showed significantly higher retention in the tumor by magnetically targeted CHL-GCS-IO NPs. (C) Infrared thermal imaging of MB49 tumor-bearing mice subjected to 808-nm NIR irradiation for 5 min. Images reveal a significant temperature rise in the GCS-IO NPs and CHL-GCS-IO NPs groups, with CHL-GCS-IO NPs (magnetic) reaching the highest temperature (~ 50.8 °C), indicating a strong photothermal conversion efficacy
The in vivo results in Fig. 8A illustrate the antitumor efficacy of CHL, GCS-IO NPs, and CHL-GCS-IO NPs in MB49 tumor-bearing mice following an i.v. injection and treatment with magnetic targeting and dual NIR irradiation (660 and 808 nm). Over the 14-day observation period, the control and CHL groups showed continued tumor growth, with visible expansion of the tumor site, as confirmed by photographic evidence. In contrast, the GCS-IO NPs and CHL-GCS-IO NPs groups exhibited significant tumor shrinkage, especially in the CHL-GCS-IO NPs group. Tumor growth in the CHL-GCS-IO NP group was effectively halted, with some tumors showing regression after treatment. The graphical representation of tumor size over time confirmed these observations, with the CHL-GCS-IO NPs group showing the most pronounced reduction in tumor size. Notably, magnetic targeting combined with the PTT and PST properties of CHL-GCS-IO NPs played a key role in enhancing antitumor efficacy compared to the other groups. Figure 8B and C further support the antitumor effects of the different treatments. At the time of sacrifice on day 14, control and CHL-treated tumors appeared significantly larger than those treated with GCS-IO NPs and CHL-GCS-IO NPs. Images of excised tumors clearly show that the CHL-GCS-IO NPs group had the smallest residual tumors, indicating strong therapeutic effects when magnetic targeting and NIR irradiation were applied. Quantitative analysis of excised tumor weights (Fig. 8C) reinforced these findings, with the CHL-GCS-IO NPs group showing a statistically significant reduction in tumor size compared to all other groups. The GCS-IO NPs group also demonstrated antitumor effects, but these were less pronounced than those of the CHL-GCS-IO NPs group, highlighting the critical role of photosynthetic oxygenation and synergistic effects of CHL in enhancing the efficacy of the NP system.
Antitumor efficacy of Chlorella (CHL), glycol chitosan iron oxide nanoparticles (GCS-IO NPs), and CHL-GCS-IO NPs in MB49 tumor-bearing mice with magnetic targeting and near infrared (NIR) irradiation. (A) Representative images of tumors in MB49 tumor-bearing mice treated with control, CHL, GCS-IO NPs, and CHL-GCS-IO NPs over a 14-day period. Tumor sizes were monitored and photographed every 2 days following intravenous administration of the formulations and subsequent magnetic targeting, and NIR 660- and 808-nm irradiation. Quantitative tumor growth curves for each group, normalized to the day 0 tumor size, are shown in the bottom panel. Data are presented as mean ± SD (n = 5). Scale bar: 0.5 cm. (B) Ex vivo images of excised tumors from all treatment groups after sacrifice on day 14. The tumor size and appearance visibly decreased in the GCS-IO NPs and CHL-GCS-IO NPs groups compared to the control and CHL groups, with the smallest tumor masses observed in the CHL-GCS-IO NPs group. (C) Quantitative analysis of sizes of excised tumors from all groups, normalized to control group’s tumor size. Data are presented as the mean ± SD (n = 5)
The enhanced antitumor activity observed in the CHL-GCS-IO NPs group can be attributed to the combination of multiple therapeutic modalities—magnetically guided targeting, PTT, ferroptosis, and PST. The presence of CHL as a photosynthetic agent likely contributed to the increased oxygen levels within the TME, helping to alleviate hypoxia and improve the efficacy of PST. The significant photothermal effect generated by NIR irradiation, as demonstrated by the temperature rise in previous figures, further amplified the therapeutic response, contributing to effective tumor ablation. In summary, Fig. 8 demonstrates the superior antitumor efficacy of the CHL-GCS-IO NPs system under combined magnetic targeting, NIR irradiation, and multimodal therapy. The ability of this system to halt tumor growth and induce significant tumor regression highlights its potential as a powerful therapeutic tool for cancer treatment. Results suggest that the combination of magnetotactic-like, photothermal, and photosynthetic effects offers a promising strategy for addressing challenges posed by the TME, including hypoxia, and improving therapeutic outcomes in cancer treatment.
Results from Figure S2 provide a detailed histological analysis of tumor and soft-tissue organs following administration of CHL, GCS-IO NPs, and CHL-GCS-IO NPs, with magnetic targeting and dual NIR irradiation (660 and 808 nm). Hematoxylin and eosin (H&E) staining was utilized to evaluate tissue morphology and potential damage caused by the treatments. The outcomes offer insights into the therapeutic effects and biocompatibility of the CHL-GCS-IO NPs system. H&E staining (Figure S2A) of tumor tissues revealed distinct morphological differences between the treatment groups. In the control group, tumor tissues maintained a highly proliferative architecture, with densely packed cells and minimal necrosis. In contrast, the CHL-treated group showed moderate signs of necrosis but retained a considerable amount of viable tumor cells, indicating a limited therapeutic effect. The GCS-IO NPs group exhibited more-extensive necrosis and a reduction in overall tumor cell density, likely due to the combined effects of magnetic targeting, PTT, and ferroptosis.
The most pronounced effects were observed in the CHL-GCS-IO NPs group, where widespread necrosis and a significant reduction in tumor cell density were evident. Tumor tissues in this group displayed a highly disrupted architecture with minimal viable cells, suggesting that the combined effects of PST, PTT, ferroptosis, and magnetic targeting effectively induced tumor cell death. Photosynthetic oxygenation provided by CHL likely contributed to alleviating hypoxia within the TME, thereby enhancing the efficacy of PST. These results highlight the superior antitumor efficacy of the CHL-GCS-IO NPs system under multimodal treatment conditions. H&E staining of major organs (Figure S2B), including the heart, liver, spleen, lungs, and kidneys, was performed to assess the potential off-target toxicity of formulationss treatments. The negative control group, representing healthy tissues, displayed a normal histological architecture in all organs. In the control and others-treated groups, organ tissues showed minimal changes, indicating that the tumor growth and CHL administration alone did not induce significant systemic toxicity. Overall, results from Figure S2 indicate that the CHL-GCS-IO NPs system effectively induced tumor necrosis without causing significant damage to healthy tissues. This biocompatibility, combined with the enhanced therapeutic efficacy, makes CHL-GCS-IO NPs a promising candidate for multimodal cancer therapy.
Fluorescence microscopic data (Fig. 9A) showed immunofluorescence (IF) staining of hypoxia-inducible factor (HIF)-1α, a key marker of hypoxia [58] and which plays a critical role in tumor progression and therapy resistance. The control group demonstrated strong HIF-1α expression, consistent with the hypoxic conditions typically observed within the TME. The CHL-treated group showed partial HIF-1α reduction, though it was moderately reduced compared to the control. In contrast, the GCS-IO NPs group also exhibited a decrease in HIF-1α expression, suggesting partial alleviation of tumor hypoxia due to PTT. Notably, the CHL-GCS-IO NPs group showed the lowest HIF-1α expression, with nearly undetectable levels of fluorescence intensity. This indicated that photosynthetic oxygenation provided by magnetotactic-like CHL-GCS-IO NPs effectively reduced hypoxia in the tumor, further supporting the enhanced therapeutic response seen in this group. The quantitative analysis (by ImageJ software) confirmed the significant downregulation of HIF-1α in the CHL-GCS-IO NPs group, which was significantly lower than in the other treatment groups.
Immunofluorescence (IF) analysis of key tumor markers in MB49 tumor tissues after treatment with Chlorella (CHL), glycol chitosan iron oxide nanoparticles (GCS-IO NPs), and CHL-GCS-IO NPs under magnetism and dual near infrared (NIR) irradiation (660 + 808 nm). (A) Hypoxia-inducible factor (HIF)-1α staining (yellow). IF images of tumor sections stained for HIF-1α, a marker of hypoxia, showing higher expression in the control and CHL-treated groups. A significant reduction in HIF-1α levels was observed in the GCS-IO NPs group, while the CHL-GCS-IO NPs group exhibited nearly undetectable levels. Quantification of the fluorescence intensity revealed significant downregulation of HIF-1α in the CHL-GCS-IO NPs group. (B) C-X-C Motif Chemokine Ligand 12 (CXCL12) (purple). IF images showed CXCL12 expression, a factor linked to tumor metastasis and angiogenesis. The control group showed high CXCL12 expression, which was moderately reduced in the CHL and GCS-IO NPs groups. The CHL-GCS-IO NPs group showed the lowest expression, suggesting suppression of proangiogenic signaling. Quantification indicated significant downregulation in the CHL-GCS-IO NPs group. (C) Transforming growth factor (TGF)-β staining (green). IF images of tumor sections stained for TGF-β, a cytokine involved in immune suppression and tumor metastasis. The control group showed high TGF-β expression, which slightly decreased in the CHL and GCS-IO NPs groups. The CHL-GCS-IO NPs group showed the most substantial reduction in TGF-β levels. Quantification confirmed significant downregulation of TGF-β in the CHL-GCS-IO NPs group. (D) Amplex red staining (red). Detection of reactive oxygen species (ROS) via Amplex red staining. The control group showed low ROS levels, while the CHL and GCS-IO NPs groups demonstrated moderate ROS production. The CHL-GCS-IO NPs group showed the highest ROS levels, indicating magnetic navigation with strong photosynthetic oxygen generation under NIR irradiation. A quantitative analysis confirmed significantly higher ROS production in the CHL-GCS-IO NPs group. Scale bar: 100 μm. Statistical significance: ns, not significant, ** p < 0.01, *** p < 0.001, **** p < 0.0001
We focused on CXCL12 (Fig. 9B), a chemokine closely associated with tumor metastasis activity and angiogenesis [59]. Similar to HIF-1α, the control group showed high CXCL12 expression, while the CHL-treated group exhibited slightly reduced levels. The GCS-IO NPs group demonstrated a further decrease in CXCL12 expression, likely due to the effects of PTT and ferroptosis. The most substantial reduction was observed in the CHL-GCS-IO NPs group, where CXCL12 expression was significantly downregulated. This suggested that the multimodal therapeutic approach not only targeted the primary tumor but may also have suppressed metastatic processes by reducing proangiogenic signals. A quantitative analysis confirmed that the CHL-GCS-IO NPs group exhibited the lowest CXCL12 levels, significantly lower than those in the other groups, highlighting the role of CHL-GCS-IO NPs in inhibiting factors that promote tumor progression and metastasis. We further evaluated transforming growth factor (TGF)-β (Fig. 9C), a cytokine involved in immune suppression and tumor metastasis [60]. The control group displayed high TGF-β expression, indicative of an immunosuppressive TME that favors tumor growth. The CHL group showed moderate TGF-β expression, while the GCS-IO NPs group exhibited a significant reduction in TGF-β levels. The CHL-GCS-IO NPs group, however, demonstrated the most profound reduction in TGF-β expression, suggesting that the combined effects of magnetic navigation with photosynthetic oxygenation and PTT induced tumor cell death and also reprogrammed the TME to a more immunologically favorable state. This shift may enhance immune-mediated tumor eradication by mitigating immune suppression. A quantitative analysis revealed that the CHL-GCS-IO NPs group had significantly lower TGF-β expression compared to all other groups. Fluorescence data (Fig. 9D) were used to detect ROS via Amplex red staining [61]; the generation of ROS is related to photosynthetic oxygen produced by CHL under light irradiation. The control group showed low ROS levels, while the CHL-treated group demonstrated moderate ROS production due to photosynthetic activity. The GCS-IO NPs group showed a further increase in ROS levels, likely due to the combined effects of ferroptosis, PTT- and PST-assisted cancer cellular ROS generation. The CHL-GCS-IO NPs group, however, exhibited the highest ROS production (p < 0.0001), confirming the strong magnetic navigation with photosynthetic activity of CHL in this group, which was further amplified by magnetic targeting and NIR irradiation. ROS generation contributed to enhanced tumor cell death and the overall therapeutic efficacy of the CHL-GCS-IO NPs system. A quantitative analysis corroborated these findings, with the CHL-GCS-IO NPs group showing significantly higher ROS levels than all other groups.
Data from Fig. 10 provide an insightful exploration of immune modulation induced by the CHL-GCS-IO NPs system, as demonstrated through IF staining of various key immune markers within the TME. Programmed death-ligand 1 (PD-L1; Fig. 10A), a major checkpoint protein often associated with immune evasion in cancer [62], was markedly overexpressed in the control group, indicating a highly immunosuppressive TME. PD-L1 expression was reduced in the CHL-treated group, showing some immune modulation, although the change was not substantial. In contrast, the GCS-IO NPs group exhibited a significant reduction in PD-L1 expression, likely due to the effects of magnetic navigation with PTT and ferroptosis, which disrupted tumor immune evasion. However, the most dramatic decrease in PD-L1 was observed in the CHL-GCS-IO NPs group (p < 0.0001), where the lowest fluorescence intensity was recorded. This indicated that magnetic navigation with multimodal treatment (PST, PTT, and ferroptosis) not only enhanced the antitumor efficacy but also suppressed immune checkpoint mechanisms, potentially allowing for greater immune system engagement against the tumor. Ferroptosis, an iron-dependent cell death driven by lipid peroxidation, is influenced by hypoxia Notably, hypoxia can influence PD-L1 expression, which has been linked to ferroptosis regulation. While hypoxia may promote ferroptosis through lipid peroxidation, it can also inhibit it via PD-L1 and GPX4 upregulation. This interplay is crucial in ischemic diseases, cancer, and immune regulation, making ferroptosis a key therapeutic target in hypoxia-related conditions [63, 64]. A quantitative analysis confirmed that PD-L1 levels in the CHL-GCS-IO NPs group were significantly lower than in all other groups, underlining the potent immunomodulatory effects of this treatment.
Immunomodulatory effects of Chlorella (CHL), glycol chitosan iron oxide nanoparticles (GCS-IO NPs), and CHL-GCS-IO NPs in tumor-bearing mice. Immunofluorescence (IF) staining and quantitative analysis of immune markers in MB49 tumor tissues after treatment with CHL, GCS-IO NPs, and CHL-GCS-IO NPs under magnetic targeting and dual near infrared (NIR) irradiation (660 and 808 nm). (A) Programmed death ligand 1 (PD-L1) expression. PD-L1 IF staining (purple) in tumor tissues showed reduced expression in the GCS-IO NPs and CHL-GCS-IO NPs groups, with the most significant reduction in the CHL-GCS-IO NPs group. A quantitative analysis confirmed significantly lower PD-L1 levels in the CHL-GCS-IO NPs group compared to all other groups. (B) Cluster of differentiation 11c (CD11c) expression (dendritic cells (DCs)). CD11c IF staining (red) in tumor tissues indicated enhanced DC activation in the GCS-IO NPs group, with the highest activation seen in the CHL-GCS-IO NPs group. (C) CD49b expression (natural killer (NK) cells). CD49b IF staining (green) revealed increased NK cell recruitment and activation, particularly in the CHL-GCS-IO NPs group, where the highest expression of CD49b was observed. (D) CD8+ T-cell infiltration. CD8+ IF staining (green) showed robust T-cell infiltration in the CHL-GCS-IO NPs group, with significantly higher CD8+ levels compared to all other groups. (E) Macrophage polarization. CD86 (M1 macrophages, red) and CD206 (M2 macrophages, green) staining showed a marked shift toward proinflammatory M1 polarization in the CHL-GCS-IO NPs group, with a significant increase in CD86 and a corresponding decrease in CD206 expression. Scale bars: 100 μm. A quantitative analysis was performed using ImageJ software, and data are presented as the mean ± SD. Statistical significance: ns, not significant; ** p < 0.01; *** p < 0.001; **** p < 0.0001
CD11c (Fig. 10B) is a marker of dendritic cells (DCs), which play pivotal roles in antigen presentation and activation of T cells. In the control group, CD11c expression was minimal, reflecting a lack of immune activation. The CHL-treated group showed a slight increase in CD11c expression, but it remained relatively low. In contrast, the GCS-IO NPs group demonstrated marked upregulation of CD11c, indicating that the treatment had begun to activate immune responses, likely driven by the magnetic navigation with inflammatory and immunogenic effects of PTT and ferroptosis. However, the highest CD11c expression was found in the CHL-GCS-IO NPs group, suggesting that the combined therapeutic magnetic navigation with modalities not only induced potent antitumor effects but also significantly enhanced DC activation. This increase in CD11c-positive cells was crucial for promoting tumor antigen presentation and initiating a strong adaptive immune response. The quantitative analysis (by ImageJ software) further supported this, showing a significant upregulation of CD11c in the CHL-GCS-IO NPs group compared to all other treatments.
Natural killer (NK) cells, marked by CD49b expression (Fig. 10C), are key players in the innate immune system and are responsible for directly killing tumor cells [66]. In the control group, CD49b expression was minimal, indicating a lack of NK cell activity. The CHL-treated group showed a slight increase in NK cell activation, while the GCS-IO NPs group exhibited a more-substantial rise in CD49b expression, reflecting enhanced innate immune activity, likely stimulated by magnetic navigation with PTT and ferroptosis. The most pronounced NK cell activation was observed in the CHL-GCS-IO NPs group, where the fluorescence intensity was significantly higher than in the other groups. This suggested that the magnetic navigation with multimodal therapy effectively recruited and activated NK cells, which may play a critical role in the rapid destruction of tumor cells. A quantitative analysis revealed a significant increase in CD49b-positive NK cells in the CHL-GCS-IO NPs group compared to all other treatments, highlighting the potent immune-stimulating effects of this system.
CD8+ T cells (Fig. 10D) are crucial in the adaptive immune response, particularly in targeting and killing tumor cells [67]. In the control group, CD8+ expression was low, indicating poor T-cell infiltration into the tumor. The CHL-treated group showed moderate CD8+ T cell activation, while the GCS-IO NPs group displayed a substantial increase in the CD8+ T-cell presence, suggesting that the treatment promoted cytotoxic T-cell recruitment and activation. The CHL-GCS-IO NPs group showed the highest expression of CD8+ T cells, indicating robust T-cell-mediated antitumor immunity. This suggested that the multimodal therapy not only induced direct tumor cell death but also enhanced the recruitment and activation of cytotoxic T cells, which can further contribute to long-term tumor control. A quantitative analysis confirmed that CD8+ T cell levels were significantly higher in the CHL-GCS-IO NPs group compared to all other treatments, demonstrating the powerful immune-stimulating potential of this system.
Macrophages (Fig. 10E) can polarize into either the proinflammatory M1 phenotype, which promotes antitumor immunity, or the immunosuppressive M2 phenotype, which supports tumor progression [68]. The control group showed high CD206 (M2 macrophage) expression, consistent with an immunosuppressive TME. The CHL-treated group showed a slight decrease in CD206 expression, while the GCS-IO NPs group exhibited a marked reduction in M2 macrophages, with a corresponding increase in CD86 (M1 macrophage) expression. However, the most profound shift in macrophage polarization was seen in the CHL-GCS-IO NPs group, where M1 macrophages were significantly upregulated, and M2 macrophages were substantially downregulated. This indicated that the multimodal therapy not only disrupted the tumor-supportive functions of M2 macrophages but also promoted activation of M1 macrophages, which are essential for initiating and sustaining antitumor immune responses. A quantitative analysis (by ImageJ software) further supported this, showing a significant increase in CD86 (M1) and a corresponding decrease in CD206 (M2) in the CHL-GCS-IO NPs group compared to all other treatments. In summary, the data presented in Fig. 10 underscore the comprehensive immune-modulating effects of the CHL-GCS-IO NPs system. By downregulating immunosuppressive mechanisms (e.g., PD-L1 and M2 macrophages) and upregulating immune-stimulating factors (e.g., CD11c, CD49b, CD8+, and M1 macrophages), this system fostered a potent antitumor immune response. The combination of PST, PTT, and ferroptosis not only induced direct tumor ablation but also reprogrammed the TME to favor immune activation, offering a promising strategy for effective cancer immunotherapy.