Characterization of MACL@UA
As shown in Scheme 1, we first synthesized MACL by the co-precipitation method, followed by MACL@UA by the one-pot method. Scanning electron microscope (SEM) images showed that the MACL presented as polygonal flakes and lamellae with dimensions mostly in the range of 600–1000 nm (Fig. 1a). Transmission electron microscopy (TEM) as well as Energy Dispersive Spectroscopy (EDS) elemental mapping revealed a homogeneous distribution of Mg, Al, and Co (Fig. 1b). Subsequently, the morphology and size of MACL were observed using atomic force microscopy (AFM), which confirmed that the MACL is a polydeformed lamellar structure with a thickness of about 1–1.5 nm (Fig. 1c). The particle sizes of the MACL and the MACL@UA were determined by a dynamic laser light scattering (DLS) analyzer, and the results were almost in good agreement with those observed by the electron microscope (Fig. 1d). We performed stability tests on MACL@UA for 7 days. The particle size of MACL@UA was stable between 650 and 780 nm and the zeta potential was stable between 6.29 and 9.51 mV (Fig. 1e). The small-angle X-Ray diffraction (XRD) patterns are shown in Fig. 1f. The MACL patterns show diffraction peaks at 2θ of 0.6°, 2.64°, and 4.94°. The wide angle XRD pattern showed diffraction peaks at 2θ of 11.72°, 23.52°32.62°, 34.68°, 39.3°, and 48.1°, indicating the successful synthesis of MACL (Fig. 1g). The presence of Mg1s, Al2p, and Co2p binding energies in the XPS pattern indicated the synthesis of MACL (Fig. 1h). ICP was used to detect the release of Mg, Al, and Co from MACL@UA (Fig. 1i). The ability of MACL@UA to release large amounts of Mg, Al, and Co lays the foundation for its ability to contribute to myogenesis and angiogenesis. Thereafter, we immersed MACL@UA in PBS to study UA release by UV-Vis absorbance of the supernatant at 280 nm (Fig. 1j). The UV-Vis standard curves for UA were determined from the peak absorbance at different the UV-Vis standard curves for UA were determined from the peak absorbance at different concentrations (Fig. 1j). We determined the encapsulation efficiency and loading efficiency of UA in MACL@UA using HPLC, which were 26.4% and 9.8%, respectively (Fig. 1k).
Characterization of MACL and MACL@UA. a) SEM images of MACL. Scale bar, 1 μm and 200 nm. b) TEM and EDS mapping results of MACL. Scale bar, 200 nm. c) AFM images of MACL and thickness measurements. Scale bar, 200 μm. d) Particle size distributions of MACL. e) Zeta potentials of MACL andMACL@UA. f) Small-angle XRD of MACL. g) Wide-angle XRD of the MACL. h) XPS spectra of MACL. i)Release patterns of Mg/Al/Co from MACL within 24 h. j)The UV-Vis absorption at 289 nm and the standard curve of UA. k)HPLC-determined encapsulation and loading efficiency of UA. (n = 3, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data are expressed in mean ± SD.)
Cell Counting Kit-8 (CCK-8) assay was performed assessing the biosafety of MACL@UA. C2C12 cells and Human umbilical vein endothelial cells (HUVECs) were co-cultured with DX for 12 h, respectively, and then sequentially added with different concentrations of MACL@UA (0, 50, 100, 150, 200, 250, 250, 300, 350, 400, 500 µg/ml)(Figure S1a). As shown, the growth of C2C12 cells was inhibited when the concentration of MACL@UA exceeded 350 µg/ml. When the concentration of MACL@UA was located between 100 and 250 µg/ml, it was growth-promoting for C2C12 cells after DX intervention (Figure S1b). The optimal growth-promoting concentration for HUVECs was located between 150 and 250 µg/ml. For macrophages, MACL@UA possessed toxicity when its concentration exceeded 400 µg/ml (Figure S1c). We also examined ER stress-related metrics in C2C12 cells and MUSCs and showed that MACL@UA did not cause ER stress (Figure S2). The results of the hemolysis assay showed that the hemolysis rate was less than 5% at concentrations of MACL@UA less than 300 µg/ml(Figure S3). Based on these biocompatibility tests, 200 µg/ml was selected as our therapeutic concentration. We also examined ER stress-related metrics in C2C12 cells and MUSCs and showed that MACL@UA did not cause ER stress.
MACL@UA reverses DX-induced myocyte senescence and promotes myocyte and vascular proliferation
To investigate the regulatory effects of MACL@UA on myocyte senescence and proliferation, C2C12 cells were selected as classical myoblasts for culture and differentiation, and dexamethasone (DX) was selected as a negative control. Four groups were established, including Control, DX, DX + MACL, and MACL@UA. Previous studies have shown that Mg2+ and Co2+ have the ability to up-regulate proliferation-related genes [33,34,35]. The results of inductively coupled plasma (ICP) also demonstrated that MACL@UA was able to release sufficient Mg2+. The results of RT-PCR illustrated that MACL@UA was able to up-regulate proliferation (Cyclin D1) as well as myogenesis (MyoG, Murf1, Myf5)-related genes (Fig. 2a) [36,37,38]. The results of Western blot also showed that MACL@UA promotes the expression of myogenesis-related proteins(Figure S4) [39, 40]. Encouraged by the up-regulation of the expression of proliferative muscle-forming related genes, the scratch assay was used to verify the effect of MACL@UA on myoblast proliferation. As shown in Fig. 2b, the addition of DX increased the scratch spacing and the addition of MACL@UA reversed the effect of DX. The results of the cell proliferation assay showed that MACL@UA not only reversed the toxicity of DX, but also promoted the proliferation of myoblasts compared to the control group (Fig. 2c, S5). Myotube formation is a hallmark of myocyte proliferation, and increased expression of myosin heavy chain gene (MYH7) implies the formation of new myotubes. As shown in Fig. 2d and Figure S6, MACL@UA not only reversed the DX-induced myotube reduction, but also increased myotube formation compared to the control group. The anti-aging efficacy of UA has been previously demonstrated. We then performed immunofluorescence staining of the different treatment groups, and the MACL@UA group down-regulated p53 and P16, which are indicators of aging, compared to the DX group (Fig. 2e, S7).
MACL@UA promotes myogenesis and vasculogenesis. a) Myogenesis and proliferation-associated mRNA expression in C2C12 cells. b) Results of scratch assay of C2C12 cells after different interventions. Scale bar, 200 μm. c) Proliferation curve of C2C12 cells. d) Immunofluorescence staining images of Myh7, an indicator of myotube formation. Scale bar, 100 μm. e) Immunofluorescence staining images of p53/p16, an indicator of senescence. Scale bar, 100 μm. f) Angiogenesis of HUVECs after different interventions. Scale bar, 200 μm. g) Quantitative analysis of total branch lenght/number of node/number of mesh results. (n = 3, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data are expressed in mean ± SD.)
Previous studies have demonstrated that cobalt ions have a stabilizing effect on the hypoxia-inducible factor (Hif-1α) [41, 42]. Therefore, cobalt ions may have a promotional effect on angiogenesis. ICP was used to detect the amount of cobalt ions released from MACL@UA. Adequate cobalt ion release provided the basis for us to investigate the effect of MACL@UA on angiogenesis. RT-PCR results all demonstrated that MACL@UA upregulated Hif-1α (Figure S8). HUVECs were used for tube-forming experiments. As shown in Fig. 2g-h, the MACL@UA group was superior to the control group in terms of total branch length, number of nodes, and amount of mesh. The cell proliferation assay also supported this result (Figure S9).
By analyzing the results of the above experiments, we found that MACL was also able to promote the proliferation and delay the senescence of C2C12. This is most likely by virtue of its excellent ability to release Mg2+ and Co2+. The ability of Mg2+ and Co2+ to promote proliferation has also been confirmed in other previous studies [37, 38, 43]. MACL@UA has a better therapeutic effect compared to MACL, most likely because UA both enhances Mg2+ and Co2+ functions and regulates C2C12 proliferation through other mechanisms. This needs to be further explored.
MACL@UA promotes AMPK and BCL-xl pathways and regulates mitochondrial homeostasis genes
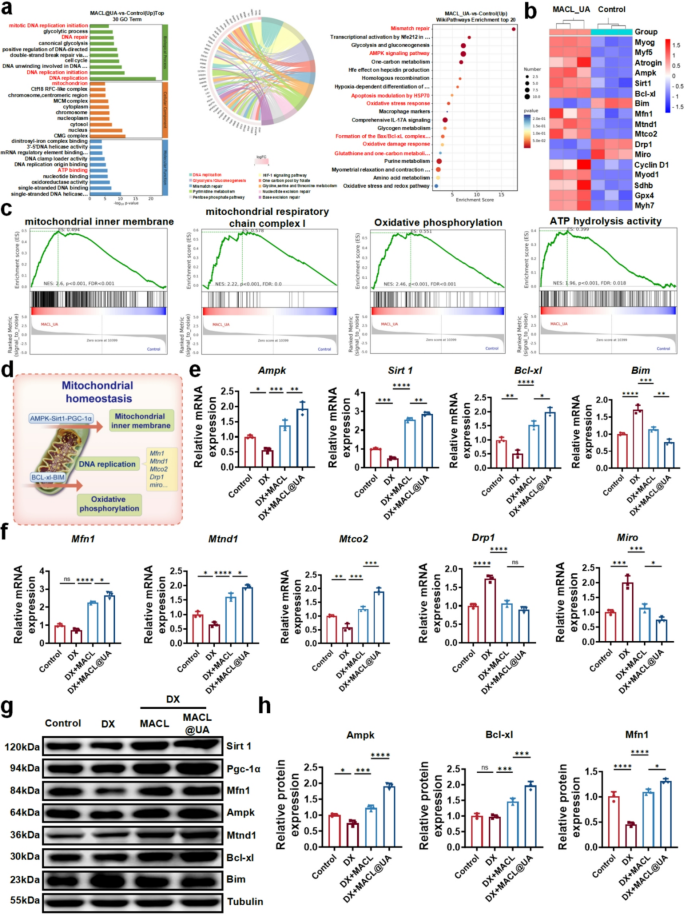
To further investigate the mechanism of MACL@UA promoting myocyte proliferation, we sequenced the whole genome of C2C12 cells from different treatment groups. Principal component analysis and correlation analysis demonstrated consistent within- group deviations, validating the RNA-seq data (Figure S10). Volcano plots showed a total of 427 genes up-regulated and 529 genes down-regulated (Figure S11). Pathway enrichment analysis showed that after MACL@UA intervention, mitotic DNA replication initiation, DNA repair, mitochondrion, ATP binding, AMPK signaling pathway and formation of BAX/ BCL-xl complex and other pathways were upregulated (Fig. 3a, S12). The results of gene heat map analysis further confirmed that MACL@UA promotes the expression of repair- and proliferation-related genes (Fig. 3b). The results of GSEA showed that mitochondrial inner membrane, mitochondrial respiratory chain complex I, oxidative phosphorylation, ATP hydrolysis activity and other pathways were upregulated (Fig. 3c, S13). The RNA-sq results demonstrate that MACL@UA not only upregulates mitotic as well as proliferation-related genes, but also promotes pathways that favor mitochondrial homeostasis (Fig. 3d). Previous studies have shown that the AMPK-Sirt1-PGC1α axis as well as the BCL-xl-Bim axis are of great significance for the regulation of mitochondrial homeostasis. Mitochondrial stability is inextricably linked to myocyte proliferation and apoptosis [44,45,46,47]. Encouraged by the RNA-sq results, we then validated the above two pathways. As shown in Fig. 3e, addition of MACL@UA reversed the down-regulation of DX for the AMPK-Sirt1-PGC1α axis as well as the BCL-xl-Bim axis, and up-regulated these pathways compared to controls. Meanwhile, DX-induced up-regulation of Drp 1 expression and down-regulation of Mfn 1 expression implies enhanced mitochondrial division, which is reversed by MACL@UA. The mRNA expressions of mitochondria motility mediator (Miro) and mitochondrial DNA (mtCO2 and mtND1) were notably changed after MACL@UA treatment. These findings altogether proved that MACL@UA could help retain mitochondrial dynamics and alleviate mitochondrial dysfunction induced by DX. (Fig. 3f) [48,49,50,51]. This result was further confirmed in Western blotting experiments (Fig. 3g-h, S14). In conclusion, MACL@UA was able to regulate mitochondrial homeostasis by up-regulating the AMPK and BCL-xl pathways and laid the foundation for the improvement of mitochondrial function.
Exploration of the Mechanism of MACL@UA to Promote Myogenesis. a) GO/KEGG/Wikipathways enrichment of up-regulated genes. b) Heat map analysis of genes for muscle formation as well as mitochondrial dynamics. c) Gene set enrichment analysis (GSEA) of mitochondrial inner membrane, mitochondrial respiratory chain complex I, oxidative phosphorylation and ATP hydrolysis activity. d) Schematic diagram of mitochondrial homeostatic regulation. e) Expression of mRNA related to AMPK-Sirt1 pathway and BCL-xl-Bim pathway in C2C12 cells. f) Related expression of mRNA of mitochondrial dynamic in C2C12 cells. g) Western blot results of the expression of mitochondrial homeostasis-related protein. h) Corresponding quantitative analysis of mitochondrial homeostasis-related protein. (n = 3, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data are expressed in mean ± SD.)
MACL@UA improves C2C12 intracellular environment and enhances mitochondrial function
Regulation of mitochondrial homeostasis is critical for alleviating myocyte injury and promoting myocyte proliferation. Elevated ROS levels due to various factors are one of the main triggers of mitochondrial homeostatic imbalance [52]. High levels of ROS are not only the cause of mitochondrial homeostasis, but also interfere with the repair of mitochondrial homeostasis. Previous studies have shown that ROS inhibit the AMPK-related pathway and thus interfere with the repair of mitochondrial homeostasis [53,54,55]. Therefore, removing ROS from the cellular microenvironment not only inhibits cellular damage, but also removes obstacles to mitochondrial repair. Intracellular oxidative stress induced by high levels of ROS is one of the important hallmarks of mitochondrial dysfunction [56, 57]. Subsequently, we examined several oxidative stress markers, including superoxide dismutase (SOD) activity [58], ROS [32, 50], and malondialdehyde (MDA) [59, 60]. Addition of MACL@UA increased SOD activity and laid the foundation for ROS scavenging (Fig. 4a). DCFH-DA was used to label intracellular ROS, and after the intervention of MACL@UA, the intracellular ROS content of C2C12 was significantly reduced compared with that of the DX group (Fig. 4b, S15). Meanwhile, the results of malondialdehyde (MDA) content further confirmed the clearance of ROS (Fig. 4c). This shows that MACL@UA is highly effective in altering intracellular oxidative stress. ATP is central to energy metabolism and supports almost all types of cellular activities, and the stability of ATP synthesis is one of the hallmarks of mitochondrial homeostasis. Our RNA sequencing results show that MACL@UA promotes the upregulation of oxidative phosphorylation, suggesting stable ATP synthesis. This result is also consistent with our subsequent Western blotting experiments (Figure S16). As shown in Fig. 4d, the addition of MACL@UA significantly increased ATP synthesis compared to the control and DX groups. Stabilization of ATP synthesis provides energetic support for mitochondrial function and repair. To assess mitochondrial function after MACL@UA intervention, we assayed mitochondrial respiratory chain complex activity. As shown in Fig. 4e, the addition of MACL@UA greatly improved the mitochondrial respiratory chain complex activity impaired by DX. Mitochondrial membrane potential (MMP) is an important parameter of mitochondrial outer membrane permeability and integrity [61,62,63]. Therefore, JC-1 was used for immunofluorescence staining to assess mitochondrial membrane integrity. The DX group showed a decrease in fluorescence intensity of JC-1 aggregates (red) and an increase in fluorescence intensity of JC-1 monomers (green), suggesting a decrease in MMP and a disruption of mitochondrial membrane integrity (Fig. 4f). Addition of MACL@UA promoted the production of JC-1 aggregates and restored the JC-1 monomer/aggregate ratio, thereby stabilizing mitochondrial membrane integrity. The regulation of MMP by MACL@UA is critical for the inhibition of cellular senescence and death. Oxygen consumption rate (OCR) is often used to reflect cellular mitochondrial function [64, 65]. Measurements of OCR showed that basal respiration was impaired by the addition of DX and improved after MACL@UA intervention. MACL@UA significantly ameliorated the reduction in maximal respiration induced by FCCP after the addition of DX (Fig. 4g). In addition, MACL@UA enhanced ATP-associated respiration. The impaired proton-leak caused by DX was also reversed to some extent by MACL@UA (Fig. 4h). The regulation of OCR by MACL@UA also underlies the promotion of myocyte proliferation. Transmission electron microscopy images showed that DX induced a decrease in mitochondrial density, cristae disruption and membrane rupture. MACL@UA ameliorated this morphological damage and increased mitochondrial density (Fig. 4i).
MACL@UA improved mitochondrial function. a) SOD activity in C2C12 cells after different treatments. b) Fluorescence images of intracellular ROS using DCFH-DA probe with various interference. Scale bar, 200 μm. c) MDA content in C2C12 cells after different treatments. d) Intracellular ATP content of C2C12 cells. e) Respiratory chain complex activity of C2C12 cells after incubation with various treatments. f) JC-1 staining image of the inner mitochondrial membrane. Scale bar, 200 μm. g) Representative oxygen consumption rate (OCR) curves. h) OCR quantification. i) TEM images of C2C12 cells mitochondria in various groups. Red arrows represent swollen mitochondria and green arrows represent intact mitochondria. Scale bar, 10 μm. (n = 3, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data are expressed in mean ± SD.)
Briefly, MACL@UA has a superior effect on regulating mitochondrial homeostasis compared to MACL. Due to the grafting of UA, MACL@UA removes high levels of ROS within the microenvironment, altering the intracellular oxidative stress environment and blocking the causative agent of mitochondrial homeostatic imbalance. ROS scavenging enhances the regulation of the AMPK-sirt1-PGC1α axis and the BCL-xl-Bim axis. It also promotes ATP synthesis by facilitating oxidative phosphorylation, which provides energy for mitochondrial function and repair. Meanwhile, the stabilization of ATP synthesis also provided energy for myocyte proliferation.The results of MMP, OCR and TEM suggested the restoration of mitochondrial function, which was indirect evidence that MACL@UA alleviated myocyte injury.
Al as a muscle-forming immune adjuvant enhances the trophic effect of macrophages on myosatellite cells
In recent years, more and more studies have shown that macrophages are indispensable in muscle regeneration [66, 67]. Factors such as Arg-1, IL-10, IGF-1, VEGF secreted by M2-type macrophages can inhibit inflammation and promote myofibril formation, vascular regeneration, and tissue reconstruction [68, 69]. UA-induced macrophage polarization to M2-type has been demonstrated in previous studies [70, 71]. Al as an immunoadjuvant has been applied to various fields such as antiviral, antibacterial, and antitumor, while studies in myogenesis are not clear. To explore the effect of Al on immune regulation, four experimental groups were established, including Control, DX, MCL@UA, and MACL@UA. The results of RT-PCR showed that MCL@UA promoted the up-regulation of genes such as macrophage Arg-1, IL-10, IGF-1, and VEGF, which was further strengthened by Al-containing MACL@UA (Fig. 5a). The results of Western blotting also showed that Al has a reinforcing role in the regulation of macrophages by MACL@UA (Fig. 5b).
MACL@UA regulates metabolic crosstalk in macrophages and myosatellite cells. a) Expression of mRNA of macrophage M2-marker. b) Western blot results of the expression and quantitative analysis of macrophage M2-marker protein. c) Macrophage intracellular levels of GLS and Gln after different interventions. d) Fluorescence staining images of GLUL. Scale bar, 100 μm. e) EDU flow analysis of macrophage supernatants after co-culture with MuSCs after different interventions. f) Quantitative analysis of EDU flow analysis. (n = 3, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data are expressed in mean ± SD.)
The metabolic interactions between macrophages and myosatellite cells are not known. However, macrophage-derived glutamine (Gln) has been shown to promote myosatellite cell activation and muscle regeneration [72, 73]. Activation of the AMPK pathway has a critical role in Gln secretion [73]. Encouraged by the activation of the AMPK pathway by MACL@UA for C2C12 cells, we examined the expression of AMPK in macrophages using RT-PCR and Western blotting. As shown in Fig. 5a-b, MACL@UA upregulated the macrophage AMPK pathway, whereas MCL@UA without Al was slightly less effective. Subsequently, we examined the levels of glutaminase (GLS), and MACL@UA significantly decreased the levels of GLS relative to the other groups (Fig. 5c). Subsequently, we found in the Gln content assay that the MACL@UA group had higher Gln content than the other groups (Fig. 5c). Immunofluorescence staining showed that the addition of MACL@UA significantly increased glutamine synthase (GLUL) secretion relative to the MCL@UA and control groups (Fig. 5d). This also provided the basis for the large amount of Gln secreted by macrophages. Finally, we collected macrophage supernatants after different treatments and added them to MuSCs for culture. JHU was selected as a Gln inhibitor for assessing cell proliferation by flow cytometry and EDU was used to label proliferating cells. As shown in Fig. 5e-f, cell proliferation was evident in the group with the addition of MACL@UA-treated macrophage supernatants, while proliferation was affected in the group with the addition of JHU.
In conclusion, MACL@UA was able to regulate the polarization of macrophages to M2 type and secrete factors such as Arg-1, IL-10, IGF-1, VEGF, which promote muscle regeneration and angiogenesis. MACL@UA was also able to activate the AMPK pathway to promote the secretion of Gln and thus nourish myosatellite cells. Our results also indicated that Al could enhance the regulatory effect of MACL@UA on macrophages.
In vivo treatment of systemic sarcopenia with MACL@UA
Encouraged by the in vitro experiments, we then explored the in vivo efficacy of MACL@UA. As shown in the Fig. 6a, systemic sarcopenia was simulated by injecting rats with a high dose of DX for 15 consecutive days, and four groups were established including Control, DX, DX + MACL, and DX + MACL@UA. We set the day the model was built as day 0, and treated the rats by intravenous injection on this day. The quadriceps muscle was taken on days 0, 7, and 14 as a representative for histologic analysis to assess the in vivo efficacy of MACL@UA. By measuring muscle width and leg circumference on days 0 and 14, we found that the muscle content of rats treated with MACL@UA increased significantly compared to DX group (Fig. 6b, S17, S18). At the same time, we weighed the removed muscles, and the muscle mass was higher in the MACL@UA group than in the other groups (Fig. 6c). We also weighed the rats during this period, and the rats in the MACL@UA group showed a significant increase in body weight compared to the DX group (Figure S19), a result that was consistent with the muscle width and leg circumference measurements, further demonstrating the excellent efficacy of MACL@UA in vivo. H&E staining of quadriceps muscle was taken on the 14th day, and it was found that the muscle tissues in the DX group were mostly degraded and necrotic, whereas the muscle tissues in the MACL@UA treatment group were rich in muscle tissue content and accompanied by the formation of blood vessels (Fig. 6d). Immunohistochemical staining of CD31 also further proved that MACL@UA had the excellent ability to promote the generation of blood vessels (Figure S20), which provided a favorable condition for the regeneration of the muscles. Myhc, an important indicator of myotube formation, was also used for immunofluorescence staining, a result that also supports the ability of MACL@UA to be used in the treatment of sarcopenia (Fig. 6e).
MACL@UA treat systemic sarcopenia in vivo. a) Establishment of myasthenia gravis model and treatment steps. b) Measurement of muscle width of different treatment groups on day 0 and 14. Scale bar, 20 mm. c) Muscle wet weight of rats in different treatment groups on day 0 and 14. d) H&E staining images of muscles and quantitative analysis of mean muscle fiber area of different treatment groups on day 14. Scale bar, 200 μm. e) Immunofluorescence staining images of myotubular markers of different treatment groups. 200 μm. f) Immunofluorescence staining images of AMPK and BCL-xl. Scale bar, 200 μm. g) Immunofluorescence staining images of different treated muscle mitochondrial TEM images. Red arrows represent swollen mitochondria and blue arrows represent intact mitochondria. Scale bar, 10 μm. h) Grip force curves of rats after different treatments. i) Distance traveled and exercise time of rats measured by treadmill experiments. (n = 3, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data are expressed in mean ± SD.)
Immunofluorescence staining and immunohistochemical staining were also used to explore the mechanism by which MACL@UA promotes myogenesis in vivo. AMPK and anti-apoptotic BCL-xl proteins were significantly up-regulated after MACL@UA treatment (Fig. 6f, S21), which suggests that the mechanism by which MACL@UA promotes myogenesis is basically the same as that in vitro. The structure and morphology of mitochondria in muscle tissue were observed by TEM, and the mitochondria in the DX group were mostly swollen with incomplete mitochondrial membranes, while the mitochondria in the MACL@UA treated group were not only structurally intact, but also increased in number (Fig. 6g).
Finally, the motor function of rats was assessed by grip test experiment and treadmill experiment. The rats showed a significant increase in grip strength on day 7, and the grip strength of the MACL@UA group was close to 350 g on day 14, whereas that of the DX group was only 290 g (Fig. 6h). The treadmill test also showed that the MACL@UA treatment significantly increased the distance and time of the rats’ exercise (Fig. 6i-j). In short, the complications of sarcopenia leading to dyskinesia can be significantly improved after MACL@UA treatment.
MACL@UA for perioperative treatment of orthopedic-related sarcopenia
Patients with systematic sarcopenia may suffer from complications such as difficulty in mobility [74], wound healing [75], and infection after orthopedic surgery. Therefore, orthopedic perioperative treatment of patients with systematic sarcopenia is crucial to avoid complications. Al has long been used as a vaccine adjuvant for antitumor, antiviral, and antibacterial applications, and whether it can be used as an adjuvant for muscle-forming vaccines remains to be explored. As shown in Fig. 7a, systematic sarcopenia was simulated by injecting rats with a high dose of DX on 15 consecutive days. The day the model was built was designated as day 0, and an intravenous injection of MACL@UA was administered, similar to vaccination. We simulated orthopedic surgery in rats on day 7 to establish a perioperative model of orthopedic-related sarcopenia, and intensive treatment with local injection of MACL@UA was performed on day 10. Thus, five groups were established, including Control, DX, DX + MCL@UA (without Al), DX + MACL@UA, and DX + MACL@UA@LI (with the addition of local injection on day 10). Measurements of leg circumference and body weight of rats in the five groups revealed that the body weight and leg circumference of the MACL@UA-containing treatment group were higher than those of the MCL@UA group and the DX group (Fig. 7b, S22–24). Muscle wet weight was higher in the MACL@UA@Li group than in the other groups (Fig. 7c). Leg circumference and body weight of the MACL@UA@LI group were even significantly higher than those of the Control group. The results of H&E staining showed that muscle fibers were more intact and abundant in the MACL@UA group compared to the MCL@UA group, suggesting that the presence of Al enhanced the role of MACL@UA in promoting muscle production (Fig. 7d). The MACL@UA@LI group had the best treatment outcome with the richest myofibers accompanied by angiogenesis. Masson staining and CD31 immunohistochemical staining results similarly confirmed that MACL@UA@LI promoted muscle repair and massive blood vessel formation (Fig. 7e, S25). By measuring the Mg2+ levels in peripheral blood, we found that after day 14, the MACL@UA group had higher peripheral hematological Mg2+ levels than the MCL@UA group (Figure S26). This phenomenon suggests that the presence of Al prolongs the residence time of MACL@UA in the body, thus providing favorable conditions for perioperative treatment. This shows that rats suffering from dystrophic sarcopenia can achieve a good muscle-forming efficacy by intravenously injecting vaccine-like MACL@UA before surgery. What’s more, local supplementation of MACL@UA at the surgical site after surgery can strengthen the effect of preoperative treatment. Geriatric sarcopenia is a systemic disease for which intravenous therapy is undoubtedly the best option, and MACL@UA, with its excellent in vivo residency, provides the rationale for long-term chronic therapy of sarcopenia. Local injections alone, while having a more efficient local therapeutic effect, lack a systemic therapeutic effect. Therefore, when confronted with the perioperative treatment of patients with systemic sarcopenia. The use of intravenous injection combined with local injection intensive treatment has absolute advantages. Intravenous injection can be used for chronic long-term treatment of senile generalized sarcopenia, and supplemented with local injection has good therapeutic effect on acute muscle loss caused by surgery.
Perioperative treatment of myasthenia gravis with MACL@UA. a) Establishment and steps of the perioperative model of sarcopenia. b) Measurement of muscle width of different treatment groups on day 0, 7 and 21. Scale bar, 20 mm. c) Muscle wet weight of rats in different treatment groups on day 0, 7 and 21. d) H&E staining images of muscles and quantitative analysis of mean muscle fiber area of different treatment groups on day 21. Scale bar, 200 μm. e) Masson staining images of different treatment groups. 200 μm. f) Immunohistochemical staining images of AMPK and BCL-xl. 200 μm. g) TEM images of muscle mitochondria after different treatments. Red arrows represent swollen mitochondria and blue arrows represent intact mitochondria. Scale bar, 10 μm. h) Grip force curves of rats after different treatments. i) Distance traveled and exercise time of rats measured by treadmill experiment. (n = 3, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data are expressed in mean ± SD.)
Immunofluorescence staining and immunohistochemical staining were also used to investigate the mechanism of myogenesis, and MACL@UA treatment promoted the up-regulation of AMPK and BCL-xl (Fig. 7f, S27–28). TEM results showed that the mitochondria were more structurally and morphologically intact after treatment with MACL@UA (Fig. 7g).
For the assessment of motor function in rats, the grip test experiment and treadmill experiment were still used. The grip strength of rats injected with MACL@UA preoperatively was significantly higher (349 g), while the main strength of rats topically supplemented with MACL@UA on the 10th day was able to reach an astonishing 370 g (Fig. 7h). Similarly, the rats in the MACL@UA@LI group were ahead of the other groups in terms of the distance and time of the exercise (Fig. 7i-j). This shows that the perioperative treatment regimen of preoperative intravenous injection of MACL@UA combined with postoperative topical MACL@UA can significantly improve the postoperative locomotor activity of rats with dystrophic myasthenia gravis.
To assess the systemic effects of intravenous MACL@UA, we performed hematological analysis on mice. The routine blood results showed that MACL@UA did not alter the levels of various types of blood cells and platelets (Figure S29). Serum biochemical analysis showed that alanine aminotransferase, glutathione transaminase and creatinine were normal after MACL@UA injection (Figure S30). MuSCs and mature muscle cells were selected for our ER stress assay, and the results showed that MACL@UA does not induce ER stress in muscle tissue in rats (Figure S31). H&E staining of major organs (including heart, liver, spleen, lungs and kidneys) showed no significant pathological changes or adverse reactions after MACL@UA injection, highlighting their high degree of histocompatibility (Figure S32).
According to the etiology and pathogenesis of myasthenia gravis, animal models of myasthenia gravis can be categorized into the following types: natural aging-related, drug-induced, exercise-deficient, nutritionally deficient and genetically engineered [76]. Laboratory animals can be used as natural aging myasthenia gravis models by keeping them in a normal environment until they reach a certain age, such as rats kept for more than 18 months. The limitations of this model are the long experimental period, the high cost, and the difficulty of controlling the differences between individuals, which may include genetic background, living environment, etc [77].
Drug-induced modeling is a common method of simulating or inducing a state of myasthenia gravis by means of a specific drug or chemical. Dexamethasone is a glucocorticoid that, when injected in high doses, inhibits the synthesis of muscle proteins and promotes their catabolism, leading to muscle atrophy. The main advantage is that aging-related changes can be observed in a relatively short period of time, but it may not fully reflect all the characteristics of natural aging [78].
Therefore, we selected rats of 18–20 months of age and injected DX at half the dose and half the course to produce a model of senile sarcopenia. Thus, we were able to maximize the control of individual differences in the natural aging-associated phenotype.