A co-cultured tumor spheroid model for in situ and dynamic detection of extracellular vesicles after immune-checkpoint inhibitor treatments
Immunotherapy includes immune checkpoint inhibitors (ICIs) designed to block checkpoint proteins, such as PD-L1 and PD-1, as depicted in Fig. 1A. The in situ detection of extracellular vesicles (EVs) is used to assess the efficacy of immunotherapy, serving as more specific biomarkers. In this study, “in situ” specifically denotes a localized EV detection method that analyzes EVs within their original 3D spheroid microenvironment, without physical disruption or isolation. Tumor spheroids, formed from a mixture of tumor cells and T cells, were generated using a self-digitization method [38, 39]. Briefly, a microfluidic chip composed of two polydimethylsiloxane (PDMS) slabs—one containing a serpentine channel and the other an array of microwells—was used to create tumor spheroids (Fig. 1B). The mixture of tumor cells and T cells was introduced into the microwells after air was expelled by a flow of fluorinated oil. Subsequently, fluorinated oil was reintroduced to shear the cell suspension in the microwells into droplets. Finally, the oil was replaced with cell culture medium for long-term culture. Using this method, an array of hundreds of cell-laden droplets was generated within the microfluidic chip. Figure 1C illustrates the workflow for ICI treatments and in situ EV detection on co-cultured spheroids. After one day of culture, the tumor cells and T cells in the droplets assembled into spheroids through intercellular connections. Drug solutions were then added to each row of spheroids for treatments of varying durations (2–7 days). A glass slide functionalized with stripes of capture antibodies, referred to as barcode antibodies, was placed perpendicular to the rows of spheroids to capture and detect the secreted EVs with an enrichment of 8–24 h. Eventually, the drug-treated spheroids in each row were dissociated into individual cells for subsequent RNA sequencing. The captured EVs from individual spheroids on the functionalized slide were detected using a fluorescently labeled CD63 detection antibody (Fig. 1D). This method enabled the determination of EV secretion levels from a single spheroid based on the fluorescent intensity of the detection antibodies immobilized on the barcode antibodies.
The captured EVs for individual spheroids appeared as a barcode stripe pattern on the slide, as shown in Fig. 1E. This approach enabled the detection of multiple EVs from hundreds of individual spheroids subjected to varying drug treatments on a single slide. By adjusting the drug treatment or EV enrichment time, we could obtain changes in EV secretion by comparing treated samples with the untreated control group. Statistical correlations were established between spheroid viability and changes in EV secretion, enabling the prediction of the viability of unknown samples for the evaluation of ICI therapies. The treated spheroids were also recovered and lysed for subsequent investigation of genomic mechanisms involved in immune pathways and gene expression.
Schematics of the formation of multicellular tumor spheroids, drug treatment, and extracellular vesicle (EV) detection. (A) Overview of the work principle. (B) Schematic of the microfluidic generation of co-cultured spheroids. (C) Workflow of the spheroid formation, drug treatment, in-site EV detection, and cell extraction for RNA sequencing. (D) Principle for EV detection using a barcode slide. (E) The main output results of this work
Immune-check point expression in the co-cultured spheroids
The spheroids generated through this self-digitization method exhibited a high degree of uniformity in both size and cell viability. MDA-MB-231 breast cancer cells and activated Jurkat T cells, used in a 1:1 ratio, were employed to establish the co-cultured spheroids as a model for tumor and immune cells, respectively. The 50% ratio of Jurkat T cells in the spheroids represents the leukocyte infiltration can account for up to 50% of cellular composition in some cancer types [40,41,42]. As a proof-of-concept study, we employed two standardized cell lines (MDA-MB-231 and Jurkat) to establish a controlled experimental system. The use of well-characterized lines provided a reproducible platform to isolate and validate the specific contributions of EVs, forming a critical foundation for future translational studies. Previous studies have demonstrated the secretion of exosomal PD-L1 by MDA-MB-231 cells and the secretion of exosomal PD-1 by activated Jurkat cells [43,44,45]. Figure 2A shows a bright-field image of the spheroids following one day of culture on the chip. The spheroid diameter exhibited high uniformity, with a coefficient of variation (CV) consistently below 0.5% across all replicates, as demonstrated by the size distribution histograms in Fig. S2 (Supporting Information). Additionally, spheroid formation efficiency reached nearly 100% in all 216 microwells per chip, ensuring reliable and reproducible 3D cell culture production. After staining with Cell-Tracker Green and Cell-Tracker Red, the viable MDA-MB-231 cells and Jurkat T cells within the spheroids are distinctly visible, as shown in Fig. 2B. The merged fluorescent image of green MDA-MB-231 cells and red Jurkat T cells in Fig. 2C further confirms the co-existence of both cell types.
To verify the formation of tumor spheroids, the cells were stained in the co-cultured spheroid after one day of culture with E-cadherin, a cell surface marker for intercellular connections [46, 47] (Fig. 2D). The cytoskeletons and nuclei of the cells were stained with Actin-Red and DAPI, respectively. The expression of Ki67 and HIF-1α indicates the cell proliferation and hypoxic state of the spheroids, respectively (Fig. 2E). The expression of cellular PD-L1 and PD-1 was confirmed by immunofluorescent staining of PD-L1 and PD-1 on the cell surface, as shown in Fig. 2F.
Morphological characterization of spheroids from MDA-MB-231 breast cancer cells and Jurkat T cells. (A) Bright-field image of a representative portion of the spheroids formed from MDA-MB-231 and Jurkat cells using the microfluidic platform. Scale bar = 500 μm. (B) Fluorescence images of co-cultured spheroids stained with Cell-Tracker Green for viable MDA-MB-231 cells and Cell-Tracker Red for viable Jurkat cells after one day of culture. Scale bar = 500 μm. (C) Bright-field and fluorescence images of the co-cultured spheroid stained with Cell-Tracker dyes. Scale bars = 100 μm. Immunofluorescence staining of spheroids after one-day culture: (D) E-cadherin (green) and Actin-Red (red); (E) Ki67 (green) and HIF-1α (red); (F) PD-L1 (green) and PD-1 (red), and DAPI (nuclei, blue), with merged fluorescence images for D, E, and F. Scale bars = 100 μm for D, E, and F
Cytotoxicity of ICIs to the co-cultured 3D spheroids and traditional 96-well plate based 2D cultured cells
To validate the co-cultured model in the evaluation of ICI treatments, we selected Atezolizumab, Nivolumab, and Tremelimumab as model drugs for anti-PD-L1, anti-PD-1, and anti-CTLA-4 therapies, respectively. For comparison, Epirubicin was chosen as a chemotherapeutic drug. The concentration of ICIs was set at 1 × 10² mg/mL to be comparable with that used in clinical ICI treatments. Epirubicin was applied at varying concentrations to the co-cultured cells in both 3D and 2D cultures.
Figure 3A presents images of MDA-MB-231 and Jurkat cells in 2D culture using a fluorescent live-dead assay after two days of various drug treatments. The green and red fluorescence in the cells identifies viable and dead cells, respectively. The tumor cells and T cells under ICI treatments show higher cell density and viability compared to those under chemotherapeutic treatments. Figure 3B shows the live-dead staining of co-cultured 3D spheroids after two days of drug treatment, with varying drug types (Fig. 3B, left panel) and varying concentrations of Epirubicin (Fig. 3B, right panel). Tumor spheroids in 3D format better mimic the TME in a more physiological condition compared to 2D monolayer cell cultures. To compare cell activity in 2D and 3D formats, the cellular viability is summarized in both culture formats after two days of drug treatments at a concentration of 1 × 10² mg/mL (Fig. 3C). Cell viability in 3D format was lower than that in 2D format for ICI treatments, while remaining similar for chemotherapeutic treatments. This may be due to the restricted space for tumor growth and higher tumor-T cell interactions in 3D format compared to the 2D format. Moreover, the viability of cells treated with ICIs was higher than that of cells treated with chemotherapeutic drugs at the same concentration in both 2D and 3D models. Figure 3D shows that cell viability decreased as the concentration of Epirubicin increased in both 2D and 3D formats, with viability being higher in the 2D culture format than in the 3D format.
Cell viability at different treatment times was also investigated. Figure 3E shows changes in cell viability in 3D co-cultured spheroids at 2, 4, and 7 days. Cell viability began to significantly decrease by day 4 for treatment of Atezolizumab and Tremelimumab, and by day 7 for treatment of Nivolumab. In contrast, cells treated with Epirubicin showed a dramatic decrease in viability at day 2 and only slight decreases at day 4 and day 7, indicating a faster cellular response to chemotherapeutic treatment than to ICI treatments. The live-dead staining and cellular viability in 2D culture at different treatment times are presented in Fig. S3 (Supporting Information). Our results reveal a pronounced reduction in cell viability within the 3D spheroid co-culture system following prolonged ICI treatment (2–7 days). In contrast, 2D monolayer cultures maintained consistently high viability throughout the treatment period (Fig. S3B, Supporting Information). This differential response may stem from potential activation-induced phenotypic changes in Jurkat cells under extended 3D co-culture conditions, and the enhanced cell-cell interactions intrinsic to the 3D spheroid architecture, which likely promote immune synapse formation and amplify cytotoxic effects. Furthermore, the high viability of MDA-MB-231 cells in 2D monolayer cultures treated with the same ICIs suggests minimal cytotoxicity of ICIs toward these cells (Fig. S4, Supporting Information).
To evaluate immune-mediated cytotoxicity, we quantified key cytokines (IFN-γ, IL-1β, and IL-6) in 3D co-culture spheroids and 2D culture supernatants after 2, 4, and 7 days of treatment with Atezolizumab, Nivolumab, Tremelimumab, or Epirubicin. The concentration of the cytokines was represented from the fluorescence intensity of the captured cytokines labeled with the fluorescence detection antibodies (Fig. S5A, supporting information). As expected, IFN-γ was undetectable in both culture systems, consistent with reports that Jurkat T cells only produce IFN-γ upon phorbol myristate acetate (PMA)/ionomycin stimulation [48, 49]. In contrast, IL-6 was elevated in both 3D and 2D cultures, while IL-1β was specifically upregulated in 3D spheroids (Fig. S5, supporting information). Cytokine secretion (IL-6 and IL-1β) declined over time with prolonged drug exposure. Notably, Atezolizumab treatment significantly increased both IL-6 and IL-1β levels compared to untreated controls, suggesting a pro-inflammatory response. Conversely, Tremelimumab reduced secretion of these cytokines, implicating an anti-inflammatory role. In contrast, cytokine secretion was dramatically reduced in both 3D and 2D cultured cells upon Epirubicin treatment, highlighting the drug’s nonspecific cytotoxicity toward immune and tumor cells.
Viability of MDA-MB-231 and Jurkat cells in 3D and 2D models after drug treatments. A. Fluorescence microscopy images of co-cultured cells stained with a Live/Dead assay after two days of drug treatments for Atezolizumab, Nivolumab, Tremelimumab, and Epirubicin at 1 × 10− 2 mg/mL and for Epirubicin at 0, 1 × 10− 5, 1 × 10− 4, 1 × 10− 3, and 1 × 10− 2 mg/mL in 2D (A) and 3D (B) formats. Scale bars in A = 100 μm, and in B = 300 μm. C. Viability of MDA-MB-231 and Jurkat cells in 3D and 2D models for two-day drug treatments at 1 × 10− 2 mg/mL. Data were analyzed from 60 spheroids and triplicates in 96-well plates for 3D and 2D models, respectively. D. Viability of MDA-MB-231 and Jurkat cells in 3D and 2D models for two-day drug treatment of Epirubicin at 0, 1 × 10− 5, 1 × 10− 4, 1 × 10− 3, and 1 × 10− 2 mg/mL. E. Viability of cells in 3D spheroids for drug treatments at 2, 4, and 7 days. Data are presented as the mean ± standard deviation. Statistical significance was determined using Student’s t-test: n.s. for p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001. One-way ANOVA test for viability grouped by the same drug in panel E: p > 0.05 for n.s. and p < 0.05 for unmarked data
Dynamic secretion of extracellular vesicles and its predictive validity of cell viability for ICI treatments
To investigate the predictive capability of EVs in evaluating ICI treatments, we developed a method to establish a correlation between the secreted EVs and the viability of co-cultured spheroids in response to drug treatments. The EVs secreted from individual co-cultured spheroids were captured by various antibodies (CD9, CD63, CD81, PD-1, and PD-L1) immobilized on a glass slide functionalized with graphene oxide quantum dots (GOQDs), as previously reported [50,51,52]. The GOQDs on the glass were used to immobilize the antibodies and minimize background noise from the fluorescent signals. Various capture antibodies were introduced through a set of parallel microchannels (20 μm in width and 30 μm in depth) above the GOQD-modified glass slide, where they were adsorbed, forming barcode-like antibody stripes. The design of the microfluidic channels for antibody immobilization and the uniformity of antibody capture on the slide were demonstrated in Fig. S1 (Supporting Information). A fluorescent IgG solution was incubated to interact with the capture antibodies immobilized on the glass slide, thereby enabling the quantification of their density. The relative standard deviation (RSD) of IgG fluorescence intensity for all capture antibodies was below 15%. This method facilitates the application of multiple capture antibodies above individual spheroids, enabling the simultaneous detection of multiple types of EVs from a single spheroid.
A fluorescently labeled antibody (CD63), a typical membrane protein on EVs [22], was incubated to bind to the captured EVs on the slide, allowing quantification of EVs based on the fluorescence intensity of CD63. Figure 4A shows the fluorescence pattern of the detection antibodies conjugated on EVs secreted from individual spheroids. To verify the capture of EVs on the barcode glass slide, the slide was imaged using scanning electron microscopy (SEM), as shown in Fig. 4B. The EVs captured on the barcode glass slide exhibited a size distribution primarily in the range of 90–400 nm in diameter, with 52% of EVs in the range < 200 nm, categorized as small extracellular vesicles (sEVs) [53]. To confirm that serum-derived EVs do not interfere with our detection, we compared EVs isolated from spheroid-containing microwells with those from cell-free microwells (Fig. S6, Supporting Information). The results demonstrate that serum EVs have negligible background effects on the detection of spheroid-secreted EVs.
To explore the temporal changes in EV secretion for individual spheroids, the EV quantity was examined for an enrichment time of 8, 16, and 24 h after two days of drug treatments. The statistical distribution of fluorescent intensity for EVs (captured by CD9, CD63, CD81, PD-1, and PD-L1 antibodies) from treatments with four drugs and the non-treated control condition is plotted in Fig. 4C (left scale). To normalize EV secretion levels, we defined a secretion change factor as the ratio between the difference in average fluorescence intensity for treated and untreated spheroids and that for untreated spheroids. The variation in secretion change factor of co-cultured spheroids treated with four drugs at different EV enrichment times is shown in Fig. 4C (right scale). A positive value of the secretion change factor indicates increased EV secretion, while a negative value indicates decreased secretion. For CD63-expressed EVs, the secretion change factor significantly increased as the EV enrichment time increased from 8 h to 24 h for all four drugs. The average fluorescent intensity and the secretion change factor of EVs secreted from the spheroids treated with different drug conditions for various treatment and enrichment durations were presented in Table S1 and S2 (supporting information), respectively. The duration of drug treatment is another factor influencing EV secretion levels. Drug treatment times of 2, 4, and 7 days were applied to co-cultured spheroids for EV enrichment of 16 h. Figure 4D (left scale) shows the statistical distribution of fluorescent intensity for the detected EVs after drug treatments of varying durations for four drugs and the control condition. The secretion change factor of co-cultured spheroids for different drug treatment times is plotted in Fig. 4D (right scale). The secretion change factor of PD-L1-expressing EVs decreased as the drug treatment time increased for all four drugs.
To explore the ability of EV secretion to predict cell viability, several machine learning models were established, including linear regression, non-linear regression, and logistic regression, for our co-cultured spheroid model using four types of drugs. The secretion change factor of EVs expressing CD9, CD63, CD81, PD-1, and PD-L1, the drug type, and the drug treatment time were defined as independent variables, and the viability of co-cultured spheroids was defined as the dependent variable. To validate our machine learning models for predicting untrained datasets, several drugs different than the ones in the training set were selected in the test set. The spheroids treated with Atezolizumab (anti-PD-L1), Nivolumab (anti-PD-1), Tremelimumab (anti-CTLA-4), Epirubicin (chemotherapeutic drug), and control were used as the training group, and the spheroids treated with Sugemalimab (anti-PD-L1), Pembrolizumab (anti-PD-1), Cadonilimab (anti-CTLA-4), Dacarbazine (chemotherapeutic drug), and control were used as the test set. The secretion change factors and cell viability for the spheroids in the test set are shown in Fig. S7 (Supporting Information).
Figure 4E shows the actual viability of spheroids plotted against predicted viability for the training and test sets using a linear regression model. The gray dotted line represents data where actual viability equals predicted viability. Each treatment condition for data points in the test set is annotated in Fig. 4E. It is found that when only the secretion change factor of PD-L1-expressing EVs and drug type were selected as independent variables, the coefficient of determination (R²) reached 0.9633 with p < 0.05 for both variables. The weights of each independent variable were estimated using a least square estimator. The estimated parameters and standard errors for each term are listed in Table S3 (Supporting Information), and the parameter for the secretion change factor of EVs carrying PD-L1 was the largest among all parameters. Therefore, the secretion change of PD-L1-expressing EVs is the optimal predictive factor for the treatment outcome of ICI and chemotherapeutic drugs in the linear regression model. Furthermore, the positive sign of the parameter associated with PD-L1 indicates a direct positive correlation between cell viability and the secretion change of PD-L1-expressing EVs. Cell viability was also predicted using a non-linear regression machine learning model with a hyperbolic tangent function and one hidden layer with 10 nodes. Figure 4F shows the actual cell viability plotted against predicted viability for the training and test sets using the non-linear regression model. The R² values for the training and test sets were 0.9839 and 0.9212, respectively, indicating better performance in predicting cell viability compared to the linear regression model. A logistic regression model was also applied to classify cell viability into two levels: 1 (0.7–1.0) and 2 (0–0.7). When only the secretion change factor of PD-L1-expressing EVs was defined as the independent variable, logistic regression showed optimal performance. The receiver operating characteristic (ROC) curves for the logistic regression model are shown in Fig. 4G. The ROC curve illustrates how the true positive rate (sensitivity) changes with the false positive rate (1 – specificity) for different cut-offs of the independent variable. The area under the curve (AUC) value indicates the model’s ability to distinguish between the two categories of viability. The AUC for viability classification was 1 for the training set (training-1 and training-2) and 0.75 for the test set (test-1 and test-2). The confusion matrix obtained from the logistic regression analysis for the training and test sets is presented in Fig. 4H.
To summarize, cell viability in the untrained test set can be predicted based on the EV secretion change factor from the training set using a linear, non-linear, or logistic regression model. Although the non-linear regression model demonstrates the best predictive performance, it is challenging to assess the contribution of each variable to the overall prediction accuracy from the non-linear regression. In contrast, linear regression provides a direct evaluation of the contribution of each variable through the estimated parameters. Both linear and logistic regression analyses revealed that the secretion change of EVs carrying PD-L1 is the most significant contributor to predicting cell viability.
Secretion change of EVs and its capability in predicting cell viability in this platform. (A) Representative fluorescence images of the secreted EVs captured and detected on a 2D functionalized glass slide for individual spheroids. The scale bars represent 300 μm for the larger image and 100 μm for the inset images. (B) Scanning electron microscopy (SEM) images of the EVs captured by the barcode slide, alongside the EV size distribution. C, D: Fluorescent intensity (left scale) and secretion change factors (right scale) of the EVs carrying CD9, CD63, CD81, PD-1, and PD-L1 secreted from co-cultured spheroids under the drug treatments with Atezolizumab, Nivolumab, Tremelimumab, Epirubicin (1 × 10− 2 mg/mL) and control conditions. Data are shown for different EV enrichment times in C and different drug treatment durations in D. E, F: The actual viability plotted against the predicted viability of the spheroids for the training set and test set using a linear regression algorithm (E) and a non-linear regression algorithm (F). The training set was derived from the average viability of spheroids under drug treatments of Atezolizumab, Nivolumab, Tremelimumab, Epirubicin, and control condition over 2, 4, and 7 days. The test set was based on the average viability of spheroids under drug treatments with Sugemalimab, Pembrolizumab, Cadonilimab, Dacarbazine, and control conditions for 4 days. G. Receiver operating characteristic (ROC) curves obtained using a logistic regression algorithm to classify cell viability into two categories: 1 (0.7-1.0) and 2 (0-0.7). H. Confusion matrix for the training and test sets analyzed by the logistic regression model shown in G
Temporal change of transcriptome expression for co-cultured spheroids under drug treatments
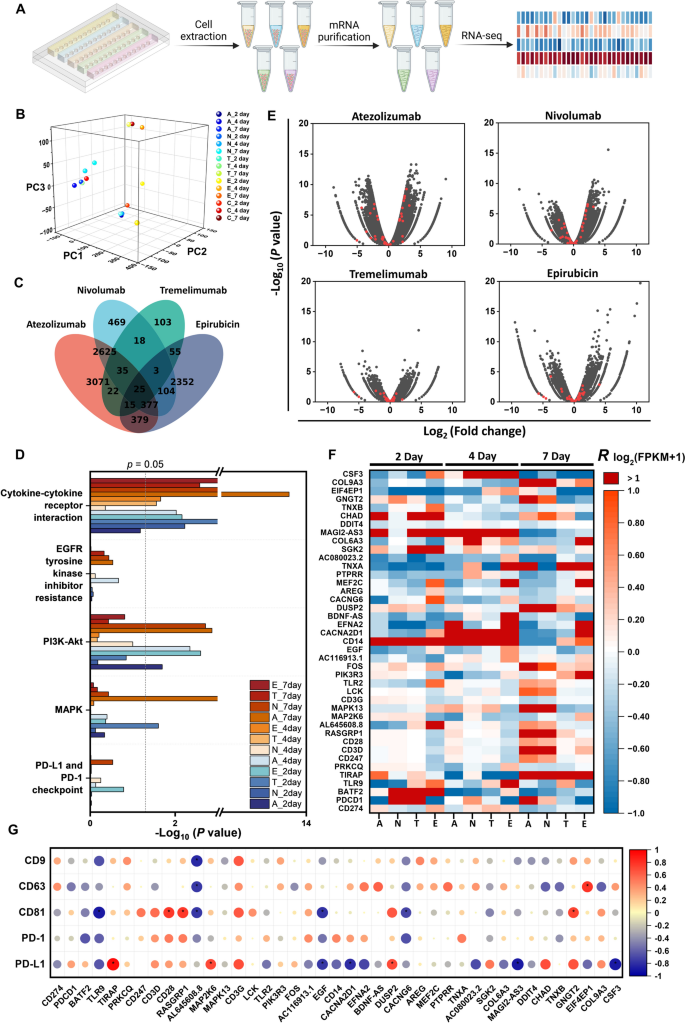
To explore the molecular mechanisms underlying the temporal changes in EV secretion after drug treatments, we performed RNA sequencing on cells from the co-cultured spheroids treated with Atezolizumab, Nivolumab, Tremelimumab, and Epirubicin, with no treatment as a control, for 2, 4, and 7 days. Figure 5A shows the workflow for sample preparation for RNA sequencing, starting from cell extraction from individual rows of spheroids under different treatment conditions. After cell lysis and mRNA purification, the sample solutions were prepared for RNA sequencing. Figure 5B presents the distribution of all samples along three dimensions based on their gene expression after Principal Component Analysis (PCA). The Venn diagram in Fig. 5C shows the differential gene expression for the four drugs compared to the control group after 7 days of treatment. The number of non-overlapping differentially expressed genes for spheroids treated with Atezolizumab, Nivolumab, Tremelimumab, and Epirubicin compared to the control condition was 3071, 469, 103, and 2352, respectively. The Venn diagram of the differential genes for the four drugs compared to the control group after 2-day and 4-day treatments is shown in Fig. S8A (Supporting Information). The correlations between different samples are plotted in Fig. S8B (Supporting Information).
To investigate the signaling pathways under different drug treatment conditions, we analyzed the RNA sequencing data via Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway clustering. We found that the pathways related to PD-1 and PD-L1 inhibition, including PD-1 and PD-L1 checkpoint (KEGG: 05235), MAPK (KEGG: 04010), PI3K-Akt (KEGG: 04151), EGFR tyrosine kinase inhibitor resistance (KEGG: 01521), and cytokine-cytokine receptor interaction (KEGG: 04060), were upregulated (Fig. 5D). The -log10(P value) of each sample indicates the activation significance of the aforementioned signaling pathways under different treatment conditions. Generally, samples treated for 7 days showed higher activation of these signaling pathways than those treated for 2 or 4 days. To visualize the regulation of the genes involved in these signaling pathways, we present volcano plots for all genes (black dots) and highlight the representative genes (red dots) involved in these pathways for the 7-day treatment samples in Fig. 5E. The volcano plots for the 2-day and 4-day treatments are presented in Fig. S8C (Supporting Information). The expression of the selected genes in these signaling pathways is shown in Fig. 5F. The expression of each gene was normalized using the R log2(FPKM + 1) method, which is defined as the ratio of the difference in log2(FPKM + 1) between drug-treated and control samples to the log2(FPKM + 1) of the control sample, where FPKM (Fragments Per Kilobase of transcript per Million mapped reads) is a normalization method for gene expression level. The FPKM values of these selected genes were listed in Table S4 (supporting information). The R log2(FPKM + 1) showed more pronounced changes for the 7-day treatment samples compared to the 2-day and 4-day treatment samples, suggesting increased activation of the signaling pathways regulating PD-1 and PD-L1 inhibition from 2 to 7 days of treatment. Specifically, the expression level of PD-L1 (CD274) decreased from day 2 to day 7, which is consistent with the trend observed in the secretion changes of EVs carrying PD-L1 (Fig. 4D).
Finally, to explore the correlation between gene expression and EV secretion, we calculated the correlation coefficients between the expression levels (R log2(FPKM + 1)) of selected PD-1 and PD-L1-related genes and the secretion change factor of EVs across five surface markers (CD9, CD63, CD81, PD-1, and PD-L1) under different treatment conditions (Fig. 5G). Among the five markers, EVs carrying PD-L1 exhibited the greatest number of significant correlations with the selected genes, highlighting the importance of PD-L1 in the regulation of PD-1 and PD-L1-related gene expressions.
RNA sequencing and differential gene expression analysis of the spheroids under different drug treatment conditions. (A) Schematic of cell isolation from the chip, mRNA purification, and RNA sequencing process used in the study. (B) The principle component analysis (PCA) of sequencing data from spheroids under treatment of Atezolizumab (A), Nivolumab (N), Tremelimumab (T), and Epirubicin (E), and non-treatment control (C) for 2, 4, and 7 days. (C) Venn diagram of differentially expressed genes among all groups for 7-day drug treatments. RNA sequencing and differential gene expression analysis revealed enrichment of 6549, 3656, 276, and 3310 genes for the treatment of Atezolizumab, Nivolumab, Tremelimumab, and Epirubicin, respectively, compared to the control group. (D) KEGG pathway clustering revealed differential presence of immune-related pathways among groups relative to the control group for different treatment conditions. (E) Volcano plots showing differentially expressed genes for all 7-day drug treatment groups relative to the control group (black dots). Red dots signify genes in immune-related pathways in D. (F) Heatmap showing expression of genes normalized as R log2(FPKM + 1) belonging to the pathways depicted in D. (G) Correlations between secretion change factor of EVs and expression levels of genes (R log2(FPKM + 1)). The color and size of the circles indicate the value of Kendall’s correlation coefficient. *p < 0.05