Janus hydrogels, with their unique asymmetric structures, demonstrate incomparable advantages over traditional materials in mucosal wet tissue defect repair. Mucosal tissues in the body (such as the oral cavity, gastrointestinal tract, and reproductive tract) exhibit complex physiological characteristics high water content (> 90%), dynamic enzymatic environment, pH gradient variations, and mucus layer barriersimposing stringent requirements on repair material performance. Wet environments cause rapid hydrogel swelling: natural polymers like collagen show swelling ratios exceeding 10 times the initial volume due to strong hydrophilic group-water interactions, accompanied by mechanical strength reduction [89]. Synthetic hydrogels may undergo volume phase transitions at the mucosal physiological temperature (37℃), compromising structural integrity. Such swelling not only accelerates hydrolysis but also shortens physical crosslinked hydrogel network disintegration time by over 50%. Janus asymmetric structural designs significantly enhance the anti-swelling performance and mechanical stability of materials. Specifically, the hydrophobic layers can effectively inhibit swelling [89]and the dense porous structures constructed through solvent exchange can greatly reduce the swelling ratio to 6.4% [90]. These design elements work together to bring about such notable improvements.
Abundant enzymatic activities in mucosal tissues (e.g., proteases, hyaluronidases) further influence material degradation behavior: trypsin hydrolyzes peptide bonds in gelatin-based hydrogels, causing 40% weight loss within 30 min; hyaluronidase activity increases 3–5 fold in inflamed mucosa, significantly shortening material lifespan. Additionally, enzymatic degradation products may trigger immune responses (e.g., chitosan oligosaccharides activating Toll-like Receptor 4(TLR4) receptors to stimulate pro-inflammatory cytokine secretion). Introducing nanoclay-limited enzymatic technology (e.g., GPC hydrogels [90]) delays degradation while enabling controlled drug release, avoiding TLR4 receptor activation by degradation products like chitosan oligosaccharides.
pH fluctuations in mucosal tissues (e.g., gastric pH 1–3 vs. intestinal pH 6–8) regulate degradation and drug release by altering material chemical microenvironments: poly (acrylic acid) hydrogels exhibit 50% reduced swelling under acidic conditions due to carboxyl protonation, while ionic dissociation accelerates swelling in alkaline environments. Janus hydrogels can integrate pH-responsive components (e.g., rhein/graphene oxide composites [91]) with zwitterionic surfaces (poly (sulfobetaine)) to achieve synergistic effects of acidic environment drug release and neutral region mucus anti-adhesion.
Mucus layer barriers (100–500 μm–thick, containing mucins andglycosaminoglycans) influence material performance through physical and chemical mechanisms: their high viscosity (1–100 mPa·s) and nanoscale network structures (10–100 nm pore size) reduce drug diffusion coefficients to 1/100th of those in water, while negatively charged glycosaminoglycans form physical barriers with positively charged chitosan. Continuous mucus layer renewal (turnover time 1–6 h) results in unmodified hydrogel half-lives < 2 h, while PEGylated surface modifications [92] (e.g., conductive polypyrrole zwitterionic layers) extend this to over 6 h, overcoming dynamic mucus renewal barriers. Additionally, mucus adsorption may trigger protein biofilm formation, impeding cell adhesion and tissue regeneration, and releasing pro-inflammatory substances like histamine. These complex environmental factors collectively present multi-dimensional challenges for Janus hydrogel design, requiring innovative material structural designs and functional integrations to precisely address multiple mucosal repair requirements. These include the need to quickly repair mucosal defects, prevent external aggression, maintain the wet environment, promote the proliferation of epithelial cells, restore the function of mucous membranes, avoid postoperative adhesion of tissues and reduce complications, provide a stable environment for tissue growth, and prevent the invasion of non-target cells, allowing monitoring of the wound. Moreover, repair materials should be able to precisely deliver drugs to the designated site and achieve the targeted loading and release of the drug during minimally invasive surgery. Conventional repair methods often struggle to meet these complex needs, and Janus hydrogels offer innovative solutions in mucosal defect repair, anti-adhesion, site-preserving functionality, stimulus monitoring, and precise drug delivery.
Defect repair function
Janus hydrogels provide an immediate physical barrier for mucosal defects by mimicking the characteristics of natural mucosal tissues, accelerating the migration and proliferation of epithelial cells, and promoting the rapid repair of the defective area. Mucosal tissue defect repair can be applied to the oral cavity, gastrointestinal tract, and other digestive tract mucous membranes. Traditional adhesive hydrogel adhesion can cause serious adhesion between damaged and normal tissues. Janus hydrogels provide a physical barrier to promote wound healing and achieve tissue adhesion under wet conditions through the properties of bioadhesives, preventing and minimizing problems such as mutual adhesion.
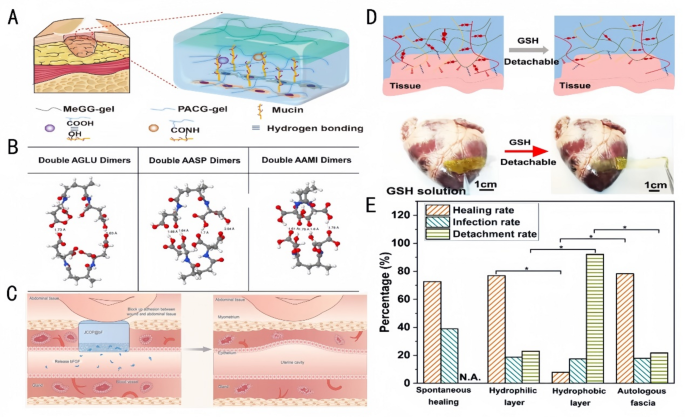
Oral ulcer (OU) is a common oral mucosal disease characterized by persistent defects in the mucosa or the disruption of epithelial integrity, thereby affecting the protective function of the mucosa [93, 94]. Materials for the treatment of OU often face problems such as poor adhesion, easy washing away by food or saliva, short adhesion time, delamination, and rapid degradation [95]. Xing et al. [96] fabricated two different functional layers of the Janus patch. One side was a smooth layer consisting of double-bonded modified junction coolant gel (Fig. 8A), which reduces non-specific adhesion and thus prevents secondary damage to the surrounding healthy oral mucosal tissue. On the other side was a Methyl Glycidyl Ether (MeGG) layer, which inhibits TGF-β1 binding to its receptor (IC50 = 0.8 µM), thereby reducing α-SMA expression by 40% and suppressing fibroblast adhesion. This helps prevent excessive fibrosis during oral tissue healing, maintaining the integrity and function of oral mucosa. The Janus patch demonstrates immediate wet adhesion with prolonged adherence time, while promoting keratinocyte migration (migration rate of 0.15 mm/h) through integrin α6β4-mediated signaling pathways. It also upregulates K14 expression (mRNA upregulation by 2.5-fold), accelerates fibroblast growth, and promotes capillary/granulation tissue formation. Withstanding oral movements such as mastication and occlusion, the patch achieves superior therapeutic outcomes. An et al. [90] reported the preparation of a Janus Gelatin-Polydopamine-nanoclay (GPC) hydrogel. This hydrogel achieved high interfacial adhesion strength and strong toughness under wetting conditions through the binding of its catechol moiety to specific functional groups (e.g., -NH2, -SH, -OH, and -COOH) on the tissue surface. In addition, the hydrogel had high cellular affinity, which facilitated cell adhesion and proliferation, thus promoting the healing of OUs. Liu et al. [97] developed a Janus hydrogel patch with excellent wet adhesion and self-debonding properties. The patch consisted of a tough layer, composed of PEGDA and PVA, to provide mechanical strength and energy dissipation, and an adhesion layer combining N-[Tris(hydroxymethyl)methyl]acrylamide (THMA) and CS to achieve strong adhesion to wet tissues by utilizing the high density of hydroxyl hydrogen bonding in THMA (Bonding strength: 8 kPa) and the topological adhesion of CS. The hydrogel promotes fibroblast proliferation (Brdu-positive rate increased by 60%) by activating the FAK/PI3K pathway, while simultaneously inhibiting MMP-9 activity (activity reduced by 55%) to reduce ECM degradation and facilitate mucosal repair. In addition, the self-unbonding property of the hydrogel helped avoid secondary damage to the repaired tissue. Chen et al. [98] also developed a thermosensitive Janus dressing based on poly(ethylene glycol)-poly(trimethylene carbonate) (PEG-PTMC) copolymers for oral ulcer treatment, achieving precise drug delivery and accelerated mucosal repair through in situ phase transition. The bilayer structure forms at oral mucosal temperature (37℃): an inner drug-loaded precipitated layer with 80 kPa adhesion strength and an outer moisture-retaining gel layer maintaining 95% hydration. Hydrophobic dexamethasone (DEX) achieves 50% cumulative release over 72 h, while hydrophilic dexamethasone phosphate (DXM-P) provides 80% burst release within 3 h, tailoring therapy for acute and chronic phases. In vivo rat models demonstrated 81% ulcer closure by day 7 (vs. 65% for Tegaderm), with 34.1 μm epithelial thickness (close to native mucosa) and 89% collagen deposition with organized fibrils. Amorphous PTMC segment ensures viscoelastic adhesion to wet mucosal surfaces, while the Janus structure provides continuous hydration and 99.8% bacterial invasion blocking. Histological analysis revealed 3 times the amount of VEGF expression promoting angiogenesis and restored nerve fibers (NF200+), indicating scarless healing. All studies addressed the challenges of insufficient adhesion and rapid drug loss in traditional oral ulcer dressings through material design. Among them, Chen et al.’s thermosensitive dressing demonstrated superior therapeutic outcomes due to its precise drug delivery and biomechanical compatibility, achieving scarless healing with 81% ulcer closure in 7 days and restoring mucosal integrity.
Hydrogel performance in adhesion and tissue repair. A: Schematic overview of the interactions between ACG and mucin [96]. Copyright 2022, Elsevier. B: The optimized conformation for double AGLU dimers, double AASP dimers, and double AAMI dimers; white, gray, blue, and red White, gray, blue, and red balls represent H, C, N, and O atoms, respectively. The dashed lines denote hydrogen bonds [100]. Copyright 2022, Elsevier. C: Application of JCOP@bF in a rat model of severe uterine injury, detailing its role in preventing adhesions and promoting JCOP@bF has a physical barrier to block adhesions and has a slow-release bFGF factor to regulate the uterine. JCOP@bF can effectively promote the recovery of the uterus and support live birth in rats [101]. Copyright 2023, Wiley-VCH GmbH. D: Schematic diagram of dissociation of a Cationized Polymethylmethacrylate(CPAMC) hydrogel triggered by GSH [91]. Copyright 2023, Nature Communication. E: Comparison of the healing, the infection, and the detachment rates of guinea pigs with TM perforations after spontaneous healing with the hydrophilic and the hydrophobic surfaces of JMs-22 and with the autologous fascia [104]. Copyright 2022, The Royal Society of Chemistry
For visceral tissue mucosal defect applications, such as gastric mucosal tissue defect repair, Liang et al. [99] achieved instant wet adhesion and anti-swelling properties as a whole by combining Polyacrylic Acid (PAA), gelatin (GT), and catechol (HBPC)-modified hyperbranched polymers, which could maintain good cohesion and adhesion as gastric perforation repair materials. Yu et al. [100] achieved good cohesion and adhesion with a gastric perforation repair material via the free radical polymerization of N-acryloylaspartic acid (AASP) (Fig. 8B). The synergistic effect of interfacial interactions and cohesive energy between the polymer molecules and the adherent surfaces was achieved by finely tuning the spatial site resistance of the polymer molecules, demonstrating adhesion strengths of up to 120 kPa. Janus hydrogel patches based on this principle achieve the properties of being adhesive yet resistant to unwanted adhesion through adhesive and non-adhesive surface bonding for wound healing and functional reconstruction and offer great potential as bioadhesives for emergency rescue and tissue/organ repair. Repair of uterine defects by the implantation of Janus hydrogel patches for tension-free healing can treat uterine anomalies and infertility. Kang et al. [101] achieved superior coverage of uterine defects and significantly improved live birth rates using the novel Janus Collagen Patch (JCOP) (Fig. 8C). With its uniform composition resembling homologous tissues, JCOP closely matches the natural uterus in structure, micromorphology and function. The rough surface and loose extracellular matrix-like porosity of JCOP promote fibroblast adhesion and endometrial tissue regeneration, while its smooth surface reduces fibroblast adhesion. The Janus structure design not only promotes the repair of damaged uteruses, restoring endometrial thickness to 89.7% of normal levels and increasing vascular density by 2.3-fold, but also restores endometrial embryo receptivity, holding significant potential for applications in treating infertility caused by uterine injury. A recent study highlights the potential of polysaccharide-based hydrogels in uterine mucosal repair. Specifically, a bilayered alginate-hyaluronic acid (Alg-HA) hydrogel fabricated via 3D extrusion-based bioprinting demonstrated enhanced endometrial regeneration in a rat model of uterine injury. This construct supported endometrial epithelial cell (EEC) monolayer formation and stromal cell (ESC) proliferation, restoring endometrial thickness and improving pregnancy outcomes. The hydrogel’s biodegradability and biocompatibility enabled controlled release of bioactive factors, fostering neovascularization and reducing fibrosis. Additionally, a 3D-printed bilayer alginate-hyaluronic acid (Alg-HA) hydrogel recently developed for uterine mucosal repair combines biocompatibility and controlled degradation to achieve sequential release of VEGF and basic fibroblast growth factor (bFGF) [102]. The hydrogel’s micro-nanoporous architecture promotes migration and colonization of endometrial epithelial cells (EECs) and stromal cells (ESCs), while simultaneously inhibiting fibrosis-related proteins via the TGF-β1/Smad signaling pathway. These mechanisms restore injured endometrial thickness to 89.7% of normal levels and enhance vascular density by 2.3-fold compared to untreated controls. These findings underscore the utility of polysaccharide-based hydrogels in addressing complex mucosal defects, such as intrauterine adhesions, by combining structural support with regenerative cues.
Myocardial infarction (MI) is one of the leading causes of death worldwide. Multifunctional hydrogel cardiac patches with Janus adhesion properties and asymmetric double-sided specific features can enable MI repair and prevent secondary trauma. For example, He et al. [91] achieved non-invasive cardiac repair and tissue adhesion prevention by Janus hydrogels, which provided mechanical support and electrical signaling in the region of MI (Fig. 8D), promoted cardiomyocyte maturation and functionalization, re-established electrical conductivity and blood supply in the infarcted area, and repaired myocardial injury.
The tympanic membrane plays an important role in the human auditory system and is prone to perforation under unfavorable conditions, leading to hearing loss and otitis media [103]. Janus hydrogels can be applied to cover tympanic membrane perforation due to their function of unilateral cell growth. Zhang et al. [104] co-deposited a tannic acid (TA)/3-aminopropyltriethoxysilane (APTES) coating on the surface of polypropylene microfiltration membrane, thus constructing Janus membranes with asymmetric cell adhesion behavior (Fig. 8E). The hydrophilic side also healed tympanic membrane perforations and restored damaged hearing. The difference in wettability between its two sides resulted in asymmetric cell adhesion properties, which prevented the repair material from adhering to the auditory ossicles, thus reducing hearing loss. Therefore, the construction of Janus hydrogels that facilitates unilateral cell growth is important for the study of new materials for tympanic membrane repair.
Based on the above literature, the mucosal repair mechanism of Janus hydrogels can be further supplemented as follows: Their bilayer structure promotes repair through synergistic effects: (1) The hydrophobic layer inhibits enzymatic degradation (e.g., pepsin) to prolong material longevity; (2) The hydrophilic layer loads growth factors (e.g., bFGF) for controlled release, accelerating epithelial cell migration; (3) The micro-nano porous structure mimics the extracellular matrix, providing a three-dimensional growth scaffold for cells. These combined actions collectively promote mucosal repair. Therefore, Janus hydrogels show great potential in the repair of various mucosal tissue defects, which can re-establish the protective barrier to prevent the invasion of external harmful factors and help restore the secretion and absorption functions of the mucosa, promote wound healing, and reduce scar formation, effectively overcoming the limitations of traditional materials. However, much is unknown about the specific physiological environment of different mucosal tissues and the differences in repair needs. In the future, we can further optimize the performance of Janus hydrogels based on the characteristics of different mucosal tissues, such as the digestive fluid environment of the gastrointestinal tract and the cyclic physiological changes of the uterus, to improve the effect of its repair. For example, for gastrointestinal mucosal repair, Janus hydrogels can be designed with acid- and enzyme-resistant properties, and at the same time, combined with growth factors that can promote the proliferation and differentiation of gastrointestinal mucosal cells to enhance the repair effect. For uterine mucosal repair, Janus hydrogels can be developed to respond to hormonal changes and promote the angiogenesis of the endometrium to better meet the special needs of uterine repair. Long-term animal and clinical studies are needed to further validate the safety and efficacy of Janus hydrogels in mucosal defect repair.
Anti-adhesion function
Postoperative wounds are often associated with the exudation of blood and tissue fluids, resulting in a moist tissue interface that is detrimental to wound repair. Moist tissue surfaces and the mutual contact of different organs in a continuous, dynamic, in vivo environment, especially in the abdomen and chest, predispose to moist tissue surfaces and organ adhesions. Janus hydrogels are effective in reducing postoperative adhesion complications by virtue of their adhesion and anti-adhesion properties because of their asymmetric structure—by facilitating tissue adhesion and at the same time preventing unwanted tissue adhesions [105].
In a rabbit model of gastric perforation, Cui et al. [106] demonstrated rapid and strong tissue adhesion in a wet environment by a Janus hydrogel with both adhesive and anti-adhesive properties. However, the other side of the hydrogel showed non-adhesive properties because the carboxyl groups were completely neutralized, thus reducing adhesion to the tissue. Thus, this Janus hydrogel could effectively prevent postoperative tissue adhesion and reduce secondary damage during surgery. This hydrogel is expected to replace traditional surgical sutures, reduce postoperative complications, and promote more effective tissue repair [107]. p(AA-co)-crylate was developed by forming a base layer from a copolymer of acrylic acid (AA) and 2-aminoethyl methacrylate (AMA), referred to as p(AA-co-AMA). p(AA-co-AMA) is a new multifunctional Janus tissue adhesive that ensures fast adhesion to wet tissues, and at the same time, provides excellent anti-adhesion properties. The anti-adhesive properties are mainly provided by a top layer of acrylic acid homopolymer (PAA) and a 2-aminoethyl methacrylate copolymer containing betaine sulfate (Zwitterionic Sulfobetaine/Aminoethyl Methacrylate Copolymer, p(AMA-co-SBMA)), referred to as AASB composition. The AASB effectively inhibits cell and tissue adhesion and reduces inflammatory responses, providing a new strategy for sutureless wound therapy and showing great potential in blocking postoperative gastric mucosal tissue adhesion. Postoperative tissue adhesions between intestinal tissues and other organs can lead to a series of complications, such as long-term pelvic pain, intestinal obstruction, and infertility, and usually require a second surgery to relieve the undesirable tissue adhesions. Moreover, current anti-adhesion biomaterials such as Interceed, Seprafilm, and anti-adhesion fluids lack tissue adhesion on the tissue-contacting side and fail to securely adhere to the tissue. Li et al. [10] effectively regulated the adhesion on the top side by complexing the GA-PAA side with PVA to form a dense and porous surface. This formation leads to a reduction in fibrinogen adsorption, with the adsorption amount dropping from 200 µg/cm² to 30 µg/cm². At the same time, it inhibits fibroblast migration, causing a 70% decrease in the migration distance. As a result, it effectively modulates the adhesion on the top surface and prevents postoperative tissue adhesion. Additionally, the abundance of carboxyl groups promotes tissue adhesion through hydrogen bonding, providing ideal adhesion for intestinal repair.
Based on the common postoperative adhesion problems after open abdominal and other surgeries, Liu et al. [108] constructed a superhydrophilic amphiphilic polymer based on a bionic microstructure. Its single-component Janus amphiphilic hydrogel patch could increase the adhesive strength through the bionic microstructure of small hexagonal surfaces separated by interconnecting grooves, and at the same time, act as a physical barrier with superior anti-adhesion effects. Liang et al. [109] utilized the porous structure and smooth bottom surface of a porous polyvinyl alcohol hydrogel (JPVA hydrogel) to reduce fibroblast adhesion, while the rough top surface improved fibroblast adhesion and tissue growth. This structure also had anti-deformation and anti-adhesion properties, which will be useful in open abdominal surgeries to reduce unwanted adhesion while enhancing adhesion to tissues. Han et al. [110]developed a Janus polypropylene mesh (PPM) via surface-initiated photopolymerization to address postoperative adhesion (PA) in hernioplasty. The mesh features asymmetric functions: one side coated with zwitterionic polymer brushes (PS) to block 99% protein adhesion and cell attachment, while the opposite side immobilizes hollow polydopamine nanoparticles (HAP) loaded with antimicrobial peptide (AMP) and platelet lysates (PLs). The PHAP layer achieves ROS-scavenging efficiency of 85%, reduces IL-6 expression by 70%, and promotes fibroblast migration (0.15 mm/h) via integrin α6β4 signaling. In vivo rat models showed 100% bacterial clearance (S. aureus/E. coli) and complete adhesion prevention (adhesion score 0/14 days), surpassing commercial meshes (score 9.7). Histological analysis revealed 89% collagen deposition with organized fibrils and three times the amount of CD31 + angiogenesis, indicating scarless healing. Li et al. [111] developed an anti-inflammatory and anti-fibrotic Janus hydrogel (PAA-Cos@ Ligustrazine (Ligu) through a composite design of cationic chitosan oligosaccharides (COS) and anionic PAA, achieving asymmetric adhesion properties on both sides. The adhesive side forms covalent bonds with tissue surfaces via carboxyl groups, while the opposite side inhibits protein adsorption through zwitterionic structures, effectively preventing peritoneal adhesions during wound repair. PAA-Cos@Ligu promotes M2 macrophage polarization and suppresses the TGF-β/Smad 2/3 signaling pathway, reducing collagen deposition and myofibroblast differentiation. In a rat model, this hydrogel fully degraded within 21 days, offering a novel strategy for clinical prevention of postoperative adhesions. Zhang et al. [112]developed a biodegradable “Janus” zwitterionic hydrogel patch for postoperative anti-peritoneal adhesion via asymmetric design: one side integrates a self-adhesive poly(acrylic acid-co-N-hydroxysuccinimide acrylate) [P(AA-co-AA-NHS)] brush layer for tissue adhesion, while the other side retains zwitterionic poly(sulfobetaine methacrylate) (PSBMA) for anti-fouling properties. The adhesive layer achieves stable wet-tissue adhesion through synergistic non-covalent (hydrogen bonding, electrostatic interactions) and covalent (NHS-amino coupling) interactions, reaching 118.07 J m⁻² interfacial toughness after 24-hour dwell time. The zwitterionic side resists protein adsorption (3.63% IgG adhesion) and fibroblast attachment (< 10% L929 cell adhesion) via hydration barrier effects. The hydrogel exhibits 0.114 MPa tensile strength, 684% elongation at break, and pH-responsive degradation (complete hydrolysis in 28 days via hyaluronidase). In a rat intestinal abrasion-abdominal wall defect model, SHAN hydrogel achieved 97% adhesion reduction (adhesion score 0.66 at 21 days) compared to PBS (4.75) and commercial HA (2.40), promoting collagen deposition (89% wound closure) while avoiding secondary inflammation. This dual-functional design addresses challenges of traditional anti-adhesion materials by integrating tissue adhesion, anti-fouling, and biodegradability, offering a promising solution for abdominal surgery.
Uterine adhesions are a common postoperative problem and can cause serious complications. Materials such as hydrogels and films suffer from poor handling, long gelling times, short residence times, and acidic degradation products [113, 114]. Fibrin deposition and fibroblast infiltration reduce the ability of materials to prevent adhesion [115]. Lv et al. [116] prepared oxidized hyaluronic Acid/methacryloylated gelatin@polycaprolactone (OD/GM@PG) bioadhesives with a wet adhesive inner layer and an anti-adhesive outer layer. These bioadhesives possessed high wet adhesive strength and interfacial toughness, downregulated the expression of inflammatory response-related proteins (S100A8, S100A9) (mRNA reduced by 50%) and inhibited NOD-like Receptor Pyrin Domain-Containing 3 (NLRP3) inflammasome activation (caspase-1 activity reduced by 60%) to reduce inflammatory exudation and prevent adhesions. Mao et al. [117] achieved a unique micro/nanopore structure and acid neutralization (The pH value rises from 4.5 to 6.8) through Janus nanofibrous barriers (GelMA-PLA/PGA/Lec). The micro/nanopore structure (The pore diameter measures 200 nm) provided excellent permeability and appropriate moisture content, which is essential to maintain barrier function and reduce adhesions. In addition, the micro/nanopore structure reduced the risk of adhesion formation by reducing fibrin deposition (70%) and resisting fibroblast adhesion (Fibroblast adhesion rate decreases to 15% (control group: 85%)). The GelMA layer neutralized the acidic environment during degradation, which helped decrease the inflammatory response and mitigate tissue damage, thereby reducing postoperative adhesions. In addition, Wang et al. [118] prepared a Janus microneedle patch using exosomes, which could penetrate the endometrium and firmly adhere to the uterine tissues while permitting the continuous release of exosomes. The tissue-adherent and anti-adhesive outer layer structure reduced the formation of tissue fibrosis, was more biologically stable, easier to store, and more efficiently delivered to target tissues [119,120,121]. The patch promoted endometrial angiogenesis and cell proliferation and increased hormonal response levels to prevent uterine adhesions, providing a new strategy to reduce postoperative uterine adhesions and promote tissue healing.
Janus hydrogels have also been investigated in the area of postoperative anti-pericardial adhesions. Cardiac surgery may lead to postoperative pericardial adhesions due to oxidative stress and inflammatory responses triggered by surgical trauma, leading to fibrinogen and collagen deposition and macrophage recruitment. Current methods of preventing pericardial adhesions suffer from weak adhesion, incomplete coverage, the need for sutures that may damage the tissue, and the possibility that the gel barrier may dissolve too quickly [122]. Wang et al. [123] reported sustained delivery of Induced Pluripotent Stem Cell-derived Cardiomyocyte Exosomes (iCM-EXOs) via injectable Janus hydrogels. These hydrogels exhibited asymmetric adhesion after photocrosslinking, acted as an antioxidant and anti-pericardial adhesion agent, effectively protected iCM-EXOs from GATA Transcription Factor, and reduced adhesions after cardiac surgery by inhibiting macrophage recruitment from the thorax.
Based on analysis of the above literature, the anti-adhesion mechanism of Janus hydrogels can be further interpreted: the non-adhesive surface inhibits tissue adhesion through three pathways: (1) polyethylene glycol (PEG) coatings create steric hindrance to block protein adsorption; (2) negatively charged surfaces (e.g., sulfonic acid groups) repel negatively charged extracellular matrix components; (3) smooth surfaces reduce mechanical interlocking. Thus, Janus hydrogels provide an effective solution to reduce postoperative adhesions and have great clinical potential. In the future, the interactions between Janus hydrogels and the surrounding tissue cells must be studied in depth to understand the molecular biology of the anti-adhesion basis, which can help further optimization of the design. In addition, we can explore the possibility of loading anti-adhesion drugs or biologically active molecules into Janus hydrogels to enhance the anti-adhesion effect or develop a biodegradable Janus hydrogel, which can avoid the need for secondary surgical removal and thus reduce the pain of patients.
Loci-saving function
Janus hydrogels have a wide range of applications in site preservation and Guided Bone Regeneration (GBR) by providing a physical barrier to protect wounds from external contaminants and infections, as well as maintaining a moist wound environment to promote tissue growth. Shi et al. [124] prepared a Janus nanocomposite hydrogel with a barrier function against fibroblast invasion, tissue preservation, and repair capability by using an osmotic cross-linking method. Its dense, smooth top surface has a significant barrier function against fibroblasts, while its loose, porous bottom surface can support bone regeneration with nanohydroxyapatite. GBR membranes isolate soft tissues from bone defects, prevent the growth of fibroblasts or epithelial cells with excessive proliferation rates, and enhance osteoblast populations to enhance bone mineralization and osseous wound occlusion [125]. However, existing GBR membranes are deficient in terms of osteogenic effect, antimicrobial properties, as well as mechanical properties and biodegradability. To overcome these limitations, Prajatelistia et al. [126] prepared a novel Janus GBR membranes. The chitin nanofiber side of this membrane promotes the proliferation and differentiation of osteoblasts. The chitosan layer binds to integrin α2β1 on the surface of osteoblasts through β-1,4 glycosidic bonds (affinity constant Kd = 0.8 µM). Meanwhile, the 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer side effectively inhibits the adhesion of fibroblasts (adhesion amount < 200 cells/cm²) and restrains the migration of soft tissues, achieving the effect of integrating the host bone tissue and creating space.This hydrogel can also activate the Bone Morphogenetic Protein (BMP)-2/Smad1 pathway (phosphorylated Smad1 increases by 3-fold). At the same time, it up-regulates the expression of Runx2 (mRNA increases by 2.5-fold) to achieve osseointegration. Chen et al. [127] reported the fabrication of Janus fiber/sponge composites using Iron Oxide Nanoparticles (IONPs, γ-\(\:{\text{F}\text{e}}_{2}{\text{O}}_{3}\)) that formed an effective barrier between alveolar bone wounds and gingival soft tissues, preventing the invasion of epithelial cells and fibroblasts from penetration. The composites exhibited superparamagnetism, which responds to changes in an external magnetic field to achieve the modulation of cellular behaviors, such as cell recruitment, proliferation, and differentiation, thereby promoting tissue repair. In addition, Wang et al. [128] effectively promoted osteoblast precursor cell adhesion, tissue repair, and in vitro angiogenesis through fibroblast-blocking ability on the dense side of the Mg-MgO/PCL Janus-structured composite membrane, as well as on the porous microfiber side through the mimetic extracellular matrix and sustained 1\(\:{\text{M}\text{g}}^{2+}\) release. Additionally, the novel bifunctional Janus GBR membrane studied by Ma et al. [129] combined a Calcium Phosphate-collagen/polyethylene Glycol (CaP@COL/PEG) layer and a Chitosan/poly(acrylic acid) (CHI/PAA) layer with a sandwich structure. This membrane exerted a barrier effect during the tissue repair process, effectively prevented the invasion of non-osteoblasts, provided favorable conditions for the formation of new bone, and effectively enhanced the effect of tissue repair. The CaP side releases Ca²⁺ at a concentration of 1.2 mM to activate the BMP signaling pathway. The PEG side inhibits fibroblast migration through steric hindrance (migration rate < 10%). Concurrently, it upregulates alkaline phosphatase activity (increased by 2-fold) and promotes the Wnt/β-catenin (Wingless/β-catenin) pathway by downregulating Dickkopf-related protein 1 (DKK1), resulting in a 40% increase in β-catenin nuclear translocation, thereby effectively enhancing tissue repair outcomes. Zhou et al. [130] studied Janus Bacterial Cellulose (BC)/MXene membranes using vacuum filtration, etching, and other techniques. They found that the dense layer played a key barrier function during tissue repair, effectively prevented the invasion of non-osteoblasts, and provided a stable space for tissue formation. In the rabbit calvarial defect model, membrane degradation time was synchronized with new bone formation (12 weeks), and the defect closure rate reached 82%. The morphology of the porous layer of the MXene nanosheets and the membrane provided a durable and stable regenerative space, together promoting tissue repair.
Janus hydrogels demonstrate their unique structural and functional advantages in site preservation and GBR by providing a physical barrier to protect wounds from external contamination and infection, while maintaining a moist wound environment to promote tissue growth. Janus hydrogels provide an effective safeguard for bone tissue repair through the combination of physical barriers, antimicrobials, and tissue-engineered scaffolds. However, the effects of Janus hydrogels on cell signaling pathways during bone regeneration are still unclear. A better understanding of the effects of Janus hydrogels on osteogenesis-related cell signaling pathways, such as Wnt/β-catenin [131], BMP [132], and other pathways, can help optimize their ability to promote bone regeneration. For example, the effects of Janus hydrogels on gene expression and protein synthesis of osteoblasts can be analyzed by gene microarray technology and proteomics methods to elucidate the molecular mechanism of increased bone regeneration. Simultaneously, by combining surface modification technology of biomaterials, bioactive molecules capable of activating osteoblast signaling pathways can be introduced on the surface of Janus hydrogels to enhance their osteoinductive properties. In addition, bone defect repair experiments in large animal models can help verify the effectiveness and safety of Janus hydrogels in the preclinical stage, laying the foundation for their clinical application.
Stimulus-monitoring function
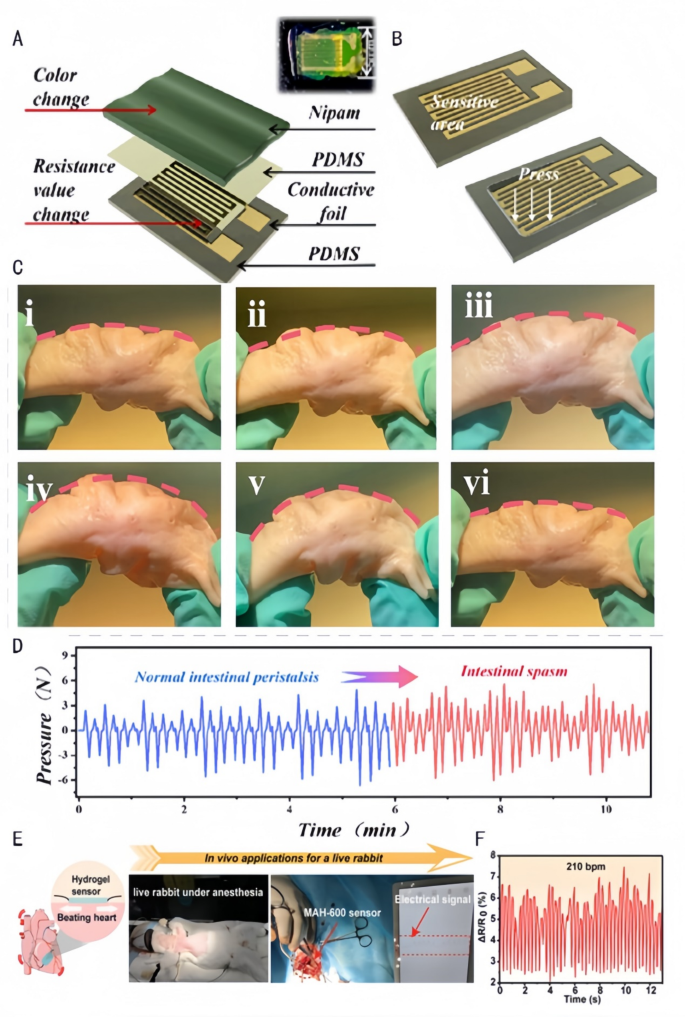
By monitoring key parameters such as temperature, humidity, perfusion, and microbial activity of the wound in real time, Janus hydrogels provide an environment that can be precisely controlled for wound healing. Chen et al. [133] combined a hydrophobic polydimethylsiloxane substrate and a hydrophilic poly(N-isopropylacrylamido-bis-acrylamidoacrylamide-acrylic acid) (P(NiPAAm-bis-AA)) hydrogel film to form a medical monitoring tool that adheres to the intestinal wall. The hydrogel regulates the mechanosensitive channel Piezo1 (mRNA increased by 3-fold) via the Phosphoinositide 3-kinase/Protein kinase B (PI3K/Akt) pathway in response to intestinal peristalsis frequency (detection range of 0.1–2 Hz). More physiological monitoring mechanism of this Janus hydrogel can be further supplemented as follows: Its ion-sensitive layer enables pressure sensing through the following pathways: (1)Temperature-responsive shrinkage/swelling alters resistance; (2)Carboxylic acid groups (-COOH) interact with ions to modulate conductivity; (3)Mechanical deformation transmits signals via microcrack propagation. This tool remains resistant to fouling and self-cleaning, forms a stable contact between the catheter and the intestinal wall and realizes the transmission of electrical signals through the integrated pressure sensor for accurate monitoring of intestinal peristalsis and evaluation of electrical signals (Fig. 9A.B). Thus, the tool can be used in diagnosing functional intestinal disorders, monitoring post-surgical recovery, and evaluating the efficacy of drugs (Fig. 9C.D). Wang et al. [105] prepared Janus hydrogels with asymmetric adhesion properties, strong wet tissue adhesion ability, and excellent mechanical toughness and electrical conductivity through the distribution of free carboxyl groups (-COOH) on both sides of the hydrogel at the interface (Fig. 9E), which can be used as highly viscous strain sensors for monitoring the in vivo heartbeat. The dynamic network is formed via thiol-ene click reaction (loss factor tanδ = 0.8), with carboxyl groups electrostatically adsorbing cardiomyocytes (adhesion amount of 800 cells/cm²). This hydrogel transmits electrical signals through connexin 43 (Cx43) gap junctions (conduction velocity of 1.5 m/s), detecting heart rate ranges of 50–250 beats per minute (bpm) with a sensitivity of 0.05 mV/bpm. The evaluation of the heartbeat in vivo can be used for the timely assessment of cardiac health, diagnosis of cardiac arrhythmia, postoperative monitoring (Fig. 9F), and the daily evaluation of exercise intensity and physical fitness.
Application of biomedical sensors in simulating intestinal motility and cardiac activity. A: Schematic illustration of the constituent layers: a PDMS substrate, a palisade conductive foil (sensitive grid), a thin PDMS layer, and a P(NiPAAm-bis- AA) layer. Inset: an opt [133]. Copyright 2024, American Chemical Society. B: External pressure causes strain on the sensor’s sensitive unit and consequently changes its resistance [133]. Copyright 2024, American Chemical Society. C: Simulation of pressure changes measured by a catheter-based pressure transducer in the porcine colon in conjunction with intestinal peristalsis. (a) A pressure-sensing catheter was placed inside the porcine colon to control the bending of the colon to simulate intestinal peristalsis. (i) Original morphology of the porcine colon; (ii) porcine colon bending by 10°; (iii) porcine colon bending by 20°; (iv) (iii) porcine colon bending by 20 [133]; (iv) porcine colon bending by 30°; (v) porcine colon bending by 40°; (vi) porcine colon returning to its initial morphology [133]. Copyright 2024, American Chemical Society. D: Image of the peristaltic pressure over time for intestinal peristalsis [133]. Copyright 2024, American Chemical Society. E&F: Normalized electrical signal of the beating rabbit heart over time detected by the MAH-600 strain sensor [105]. Copyright 2023, Wiley-VCH GmbH
Janus hydrogels show great potential in the monitoring of human intestinal motility and cardiac physiology. By combining hydrophobic and hydrophilic material characteristics, they achieve safe adhesion to the intestinal wall and maintain stain resistance and self-cleaning properties, providing a stable and intuitive solution for intestinal monitoring. This asymmetric adhesion property combined with excellent mechanical and electrical conductivity can be used as a strain sensor to accurately monitor physiological activities such as the heartbeat, providing a new technological means for human health monitoring and disease prevention. At the same time, Janus hydrogels provide real-time feedback on the changes in the internal environment of the organism by monitoring the physiological signals during the tissue healing process, evaluating the effects of tissue repair and regeneration, adjusting the treatment plan in time, and optimizing the repair strategy. However, there is still room for improvement in the sensitivity and accuracy of Janus hydrogels. In the future, their monitoring performance can be improved through material innovation and sensor technology optimization, such as introducing nanomaterials to enhance the responsiveness or accuracy of the sensor. In addition, Janus hydrogel sensors can be developed for multi-parameter monitoring, simultaneously monitoring tissue temperature, humidity, pH, inflammatory factors, and other indicators, thus providing richer data for a comprehensive understanding of the healing process. Janus hydrogel sensors can also be combined with wireless transmission technology to achieve remote real-time monitoring, which is convenient for healthcare professionals to keep abreast of changes in the patient’s condition and improve the efficiency of medical care.
Precision transport
Although endoscopic surgery has been widely used for minimally invasive procedures, the use of hydrogel membranes during minimally invasive procedures is limited by the difficulty in spreading them to completely cover irregular or folded tissue surfaces [134]. Conventional hydrogels may break or self-adhere endoscopically during minimally invasive procedures. In contrast, due to their structure, Janus hydrogels can be delivered stably through the endoscope. Moreover, the different wettability, pore structure, and chemical composition of the two sides of Janus hydrogels allow for the targeted loading and controlled release of drugs. This smart responsiveness allows the Janus hydrogel to modulate the drug release rate according to the changes in the surrounding environment (e.g., pH, temperature, or ion concentration), enabling precise delivery [7]. Meanwhile, the good mechanical strength and flexibility of Janus hydrogels ensures the stability and fit of the delivery system in complex biological environments.
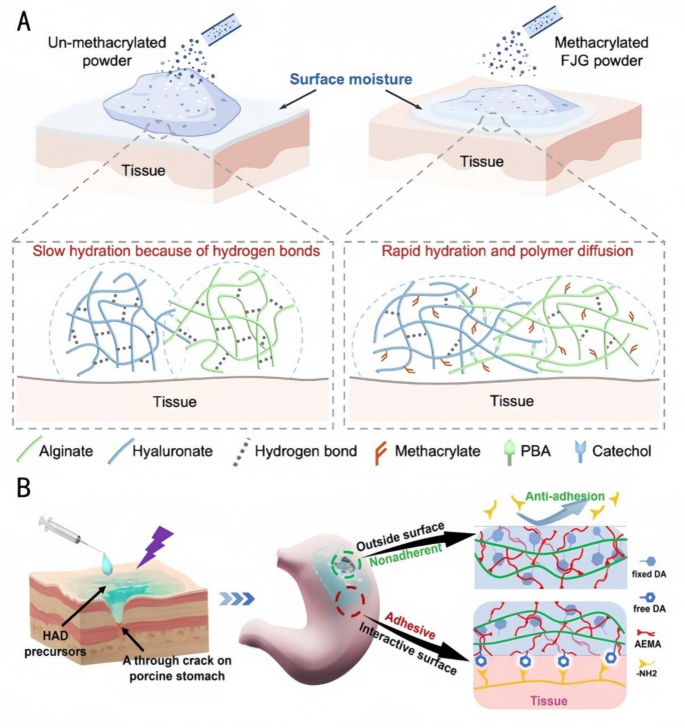
Investigating the use of Janus hydrogels in endoscopic surgery, Jia et al. [135] prepared Fast Gelation (FJG) powders with rapid water absorption and fast gelation capabilities (Fig. 10A). They modified the polysaccharide macromolecules by methacrylation to improve their water absorption ability and by using the rapid dynamic addition of borate bonds for fast gelation after hydration. The main components were the polysaccharide macromolecules modified by methacrylamide macromolecules, and the upper powder was cationized by treatment with a cationic chitosan solution to obtain a non-adhesive Janus hydrogel on the upper surface. The Janus hydrogel formed by FJG powder exhibits favorable viscoelasticity and an appropriate in vivo degradation rate. It can be delivered through a 2.8 mm biopsy channel, covering 100% of the gastric perforation area (5 mm in diameter). This hydrogel effectively prevents postoperative adhesions while also featuring easy storage and low-cost delivery. Wu et al. [136] fabricated an injectable Janus hydrogel with injectable asymmetric adherent hydrogels (HADs) via a photocuring technique and a Minimally Invasive Delivery (MID) device [137]. This hydrogel enabled precise delivery and rapid prevention of fluid leakage in laparoscopic surgery through the sealing and wound-healing capabilities of the hydrogel on the inside (Fig. 10B), the anti-adhesive properties of the outside, and the use of special syringes for MID and precise injection. It overcomes the problem of most hydrogels being preformed in patch form, lacking the ability to gelate in situ, and having limited application in minimally invasive surgery for gastric perforation. Based on the systematic summary of the above literature, the precise drug delivery mechanism of Janus hydrogels can be deduced as follows: Their asymmetric structure enables targeted release via three pathways: (1) The hydrophilic layer adsorbs drugs and triggers pH-responsive release; (2) The hydrophobic layer drives unidirectional drug transport using capillary action; (3) Photothermal-responsive materials (e.g., PDA) accelerate drug release under near-infrared irradiation. These synergistic effects thereby achieve precise drug delivery.
Applications of FJG powder and HAD hydrogels in tissue engineering. A: Schematic diagram of hydration of FJG powder [135]. Copyright 2023, PNAS. B: Schematic diagram of the Janus HAD hydrogel for robust and efficient sealing of stomach tissues: The inner-side surface of HAD hydrogels could form robust adhesion on the stomach surface due to a Michael-type reaction, while the outward-side face of HAD was nonadherent due to the restriction of free DA groups after the Michael-type reaction post-photocrosslinking [136]. Copyright 2023, Theranostics
Membrane hydrogels are difficult to adapt to irregular tissue surfaces, and stability issues during surgery are difficult to resolve [114]. Janus hydrogels not only provide a solution for good tissue compliance, easy storage, and low-cost delivery in endoscopic surgery, but also enable precise delivery and rapid fluid sealing during surgery through light-curing technology and MID devices. These improved approaches not only improve the therapeutic efficacy of the procedure, but also open up new possibilities for Janus hydrogels to be used in clinical procedures in minimally invasive surgeries, thereby improving the patient’s surgical experience and recovery process. However, there are still some pressing issues that need to be addressed in the practical application of Janus hydrogels. Currently, little is known about how Janus hydrogels can be precisely shaped at the desired site and achieve effective drug release in the complex in vivo environment. Most studies are limited to in vitro experiments or simple animal models and lack in-depth investigation in the human physiological environment, making it difficult to accurately assess the actual effects of Janus hydrogels on different individuals and complex diseases. When targeting irregular tissue surfaces, it is difficult to ensure the fit of hydrogels, which may result in some tissues not being effectively treated, affecting the overall efficacy [116]. Moreover, because the synergistic relationship between the hydrogel molding process and drug release is unclear, precise delivery of Janus hydrogels is challenging. In view of these challenges, in the future, imaging technologies, such as magnetic resonance imaging and computed tomography, combined with in vivo tracer technology, can be used to monitor the delivery process, the molding location, and the dynamics of drug release from Janus hydrogels in vivo in real time. This will help gain insights into the mechanism of their behavior in complex physiological environments and provide a basis for the optimization of their design. Through material modification and structural optimization, Janus hydrogels can be designed with self-adaptive ability so that they can automatically adjust their morphology according to the shape and characteristics of the tissue surface and improve the fit on irregular tissue surfaces and the uniformity of drug release.
Other
The versatility of Janus hydrogels allows for more than just the above mentioned biomedical applications. For example, Janus hydrogels are also widely used in the anti-infection of the nasal mucosa. The nasal cavity is highly susceptible to postoperative infections and recurrent inflammation due to spatial and environmental constraints. Commonly used postoperative dilatation sponges can only isolate the wound but not fight infection or accelerate wound recovery. To promote rapid healing of the nasal cavity, Luo et al. [17] prepared a multifunctional amphiphilic wound dressing nanofibrous material with Janus superhydrophilic/superhydrophobic capability by using PCL-gelatin fibers as a pump-absorbent layer. The superhydrophilic pump-absorbent layer maintains the moist environment of the wound by absorbing and isolating the wound exudate, and meanwhile, the RGD sequence promotes integrin αvβ3 binding (Kd = 1.2 µM), activates the Focal Adhesion Kinase/Phosphoinositide 3-Kinase (FAK/PI3K) pathway (phosphorylation levels increased by 3-fold), reduces IL-8 secretion (concentration decreased from 200 pg/mL to 50 pg/mL), and upregulates IL-10 expression (increased by 2-fold), thereby effectively blocking bacterial invasion. The superhydrophobic layer prevents bacterial adhesion and colonization, thus effectively preventing infection. Lei et al. [138] prepared triple-toluene-hardened (MTS)-based Janus hydrogels (MTS@P/DLT) with asymmetric adhesion (MTS@P/DLT) through the use of flexible wood as the skeleton and PVA as the outer matrix. One side has The viscosity of the lower double-layer thiol-enclosed click chemical film (Dual-Layer Thiol-ene Click Chemistry Film, DLT), which is important for the flexibility and anti-adhesion properties of the hydrogel, while the other side has a higher viscosity. Compared with ordinary hydrogels, Janus hydrogels have bacteriostatic properties and their increased flexibility in response to the constant movement of the nasal mucosa enhances the degree of adhesion to the tissues, creates a sterile and invasive environment, and reduces the risk of recurrence of rhinitis.
Janus hydrogel drug transport capacity has applications in anticancer. Lee et al. [139] constructed Janus polysaccharide membranes composed of Chitosan-catechol (Chi-C), which is a strong adherent, and alginate (Alg), which is an anti-adherent. The formation of a stable bilayer structure with electrostatic interactions enables the control of differences in the strength of tissue adhesion, with the strong adhesion of the Chi-C layer ensuring tight binding of the film to the tissue, and the weak adhesion of the Alg layer providing the necessary anti-adhesive properties. This Janus membrane can encapsulate the anticancer drug doxorubicin (DOX). The Alg layer releases DOX in an acidic tumor microenvironment (pH 6.0) with a release rate of 90%, while only < 15% is released in a neutral environment (pH 7.4). DOX inhibits Topoisomerase II activity (enzyme activity reduced by 80%) by intercalating into DNA base pairs (Kd = 0.1 µM) and activates caspase-3/7 (activity increased by 4-fold), inducing tumor cell apoptosis (apoptosis rate of 75%). Meanwhile, released DOX promotes dendritic cell (DC) expression of CD80/CD86 (increased by 3-fold), activating CD8 + T cell responses. Mannose residues in the Alg layer promote M1 macrophage polarization via MR receptors (CD86 + cells increased by 60%). This material has a DOX loading capacity of 8% (w/w), an encapsulation efficiency of 92%, accumulates drug concentrations at the tumor site 8-fold higher than in normal tissues, achieves a 89.7% tumor volume inhibition rate over 21 days, and exhibits no significant systemic toxicity, providing an efficient and safe solution for tumor-targeted therapy. Additionally, thiolated chitosan-lithocholic acid nanomicelles for ergotamine delivery [140] highlight a novel strategy for enhancing mucosal adhesion and pH-triggered drug release. This system demonstrated 89.7% tumor reduction in murine models through dual-functional mechanisms: thiolation-enhanced mucoadhesion and acid-responsive drug release at ~ pH 5.5 (close to that of tumors. Such innovations could inspire Janus hydrogel designs with asymmetric layers—one optimized for targeted drug delivery via pH-responsive nanocarriers, and the other engineered for antimicrobial or anti-adhesive properties—to minimize systemic toxicity while maximizing local therapeutic outcomes.
The design and application of these innovative materials have not only improved therapeutic efficacy but also reduced the risk of postoperative complications and improved the quality of life of patients. However, the physical and chemical properties of Janus hydrogels must be further optimized to achieve a targeting effect on specific cells or tissues, improve therapeutic effect, reduce damage to normal cells, and promote tissue repair. For example, the effect of Janus hydrogels on nasal mucosa cells can be observed in the long term, in addition to determining whether their degradation products in the nasal environment will cause allergy or other adverse reactions. When used for anticancer applications, it is necessary to study the distribution and metabolism of Janus hydrogels in tumor tissues to assess their toxicity and potential side effects on normal tissues. In addition, the potential of Janus hydrogels in the treatment of other diseases, such as ophthalmic diseases and neurological diseases, can be further explored to provide more therapeutic options for the biomedical field.