Synergistic induction of robust CCL4 production via β-Catenin suppression by “NCTD + lomi” synergism
Despite the exponential increase in studies using nanocarriers to co-deliver drug combinations since 1999 [25], most research based on this de novo approach remains confined to preclinical stages, with Vyxeos® being the only approved example to date [26]. In this context, we sought to explore the underlying potentials of the “NCTD + lomi” regimen, both of which are already marketed. NCTD has been shown to suppress TNBC cancer stem cell (CSC)-like properties by deactivating β-catenin pathway [24]. However, its single use does not satisfactorily enhance CCL4 expression in tumor cells. Given that antineoplastic medicines with similar effects are often administered in combination, small molecules eradicating CSC-like properties were presumed to assist NCTD in restoring CCL4. From a library of FDA-approved drugs, the lipid-lowering agent lomi was found to have the potential to attenuate CSC-like properties [23] and was thus utilized as an adjunct to NCTD.
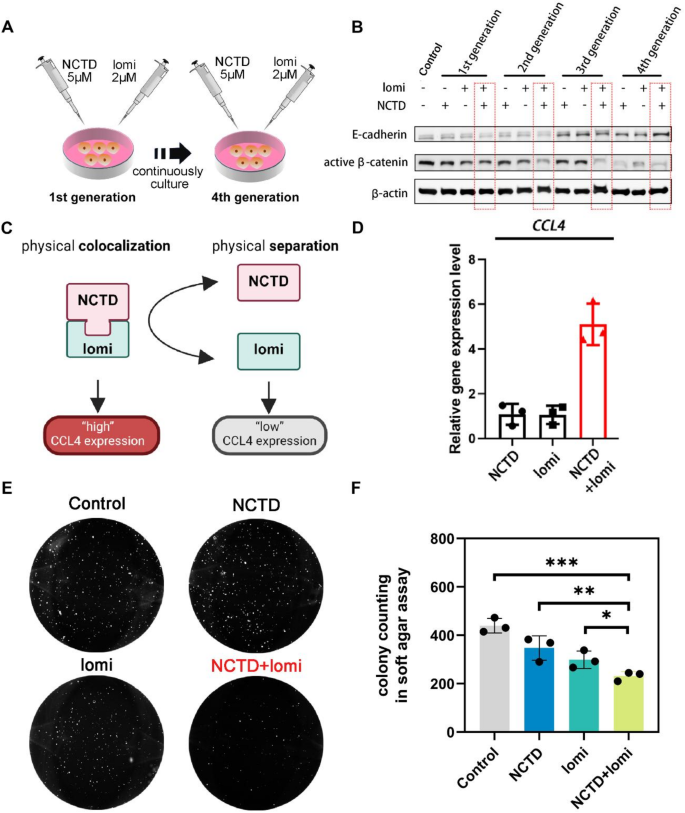
Regardless of the use of radiation, chemotherapy, or immunotherapy, TNBC features a poor prognosis and low five-year survival rate, and is deemed an inflamed-cold tumor. The mouse TNBC 4T1 cell line was chosen as the cold tumor model, which exhibits limited immune infiltration and low response to anti-PD1 treatment [27]. As shown in Fig. 2A, the “NCTD + lomi” combination strategy was designed to treat 4T1 cells over four generations continuously. Compared to single-agent treatments, the combination showed impressive synergism, diminishing active β-catenin levels from the 2nd generation and enhancing E-cadherin levels by the 4th generation (Fig. 2B). This was further validated by quantitative analysis of Western blot (WB) data from three independent experiments, with the results represented in the column charts (Supplementary Fig. 1). The data consistently showed significant reductions in active β-catenin and increased E-cadherin expression in the combination treatment group, highlighting the enhanced therapeutic efficacy. Furthermore, we utilized MSAB, a small molecule known to directly bind β-catenin and promote its proteasome-mediated degradation, thereby specifically inhibiting the Wnt/β-catenin pathway. Previous studies have demonstrated that MSAB can effectively suppress the growth of Wnt/β-catenin-dependent cancer cells, highlighting its potential as a therapeutic strategy [28]. To further investigate the mechanism underlying the enhanced antitumor effect of combination of NCTD and lomi, we performed WB analysis to evaluate β-catenin signaling. The results revealed that cells treated for four generations with NCTD and lomi markedly reduced the expression levels of both total and active β-catenin. Notably, this inhibitory effect was comparable to that of MSAB (Supplementary Fig. 2). These findings suggest that combination of NCTD and lomi may suppress tumor progression, at least in part, by modulating the Wnt/β-catenin signaling pathway, in a manner similar to MSAB. To investigate whether the treatment affected the cancer stem-like cell population, we evaluated the mRNA expression levels of CD44 and CD24, two well-established markers used to characterize breast cancer stem cells. In particular, the CD44⁺/CD24⁻/low phenotype has been widely associated with breast cancer stemness, self-renewal, tumorigenicity, and therapeutic resistance [29]. The expression of CD44 was significantly reduced, while CD24 expression was elevated after treatment, leading to an increased CD24/CD44 ratio (Supplementary Fig. 3). This trend suggests a reduction in the stemness phenotype of breast cancer cells. The shift in the CD24/CD44 ratio suggests a reduction in stem-like characteristics, which could indicate a decrease in the cancer stem cell population after treatment. Both proteins were important markers for cancer stemness, and thus “NCTD + lomi” elicited more robust CCL4 expressions than its individual components (Fig. 2C). Preliminary evidence indicates that the combined use of the two drugs results in a five-fold increase in the expression of the chemokine CCL4 compared to the use of either drug alone (Fig. 2D). Inappropriate activation of the Wnt pathway drives tumor formation [30]. Similarly, the “NCTD + lomi” combination also exhibited a strong inhibitory effect on three-dimensional (3D) soft agar long-term colony formation by 4T1 cells (Fig. 2E, F), implicating reduced oncogenesis of individual CSC at the distant organ. As β-catenin activation is strongly associated with CSC-like properties, the results revealed a deactivated β-catenin status due to the combination treatment. Immune evasion in many cancers is also associated with β-catenin activation, which inhibits cytokines/chemokines gene expression. Chemokine CCL4 plays a critical role in recruiting immune cells, including CD8+ T cell infiltration [1, 31, 32]. Thus, based on these pilot study results, we proposed a small molecule strategy utilizing NCTD and lomi to elicit CCL4 expression in TME.
In a combinatorial regimen, the efficacy magnitude is generally depicted as either simple additivity or superadditivity (synergism). Traditionally, the primary role of developing co-delivery NPs has been to avoid the chemoresistance of tumor cells through various mechanisms, with synergism among drugs not being the top priority [33, 34]. However, in our case, the super synergism demonstrated by the “NCTD + lomi” combination far exceeded from their individual potencies. This suggests that their efficacy in deactivating β-catenin will diminish after NP physical disassociation, thereby minimizing impairment to somatic stem cells. Collectively, this level of synergism provided an excellent opportunity to modulate CCL4 expression by combining or separating the molecule pair.
The synergism of two small-molecule combination to elicit CCL4 expression via deactivating β-catenin. (A) Schematic illustration of NCTD (5 µM) and lomi (2 µM) co-exposure from the 1st generation 4T1 cells to the 4th generation 4T1 cells. (B) Western blotting results of E–cadherin and active β-catenin levels of 4 generations 4T1 cells after lomi + NCTD co-exposure. (C) Schematic representation illustrating the synergistic effect of NCTD and lomi on the regulatory mechanism of CCL4 expression. (D) qPCR analysis of CCL4 mRNA expressions on 4T1 cells after exposure to the single- and dual-drug. (E) Colony formation of 4T1 cells on soft agar. Anchorage-independent cell growths from control, NCTD, lomi and NCTD + lomi groups were evaluated by soft agar assay. The representative pictures were presented by Colony formation of 4T1 cells on soft agar. (F) Quantitative analysis of 4T1 cells colony formation. The data were presented as mean ± SD (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001
Design and assessment of NCTD-lipid prodrugs
NCTD has antitumor effects due to its structure, which contains an oxygenated six-membered ring and dicarboxylic anhydride. However, it is prone to ring opening and hydrolysis in the aqueous environment, ultimately transforming into NCTD diacid. The limited half-life of NCTD diacid restricts its potential applications in cancer therapy [35]. In contrast, lomi is a hydrophobic molecule with a water solubility as low as 5.267 × 10− 8 mg L-1 [36]. The drastic differences in their physiochemical properties can lead to the premature release of NCTD, which may compromise the synergism needed to restore chemokine CCL4 in vivo. Precisely controlling the release profile of NCTD to match that of lomi is important. To optimize these unfavorable physicochemical properties, a prodrug strategy was adopted for NCTD in present study.
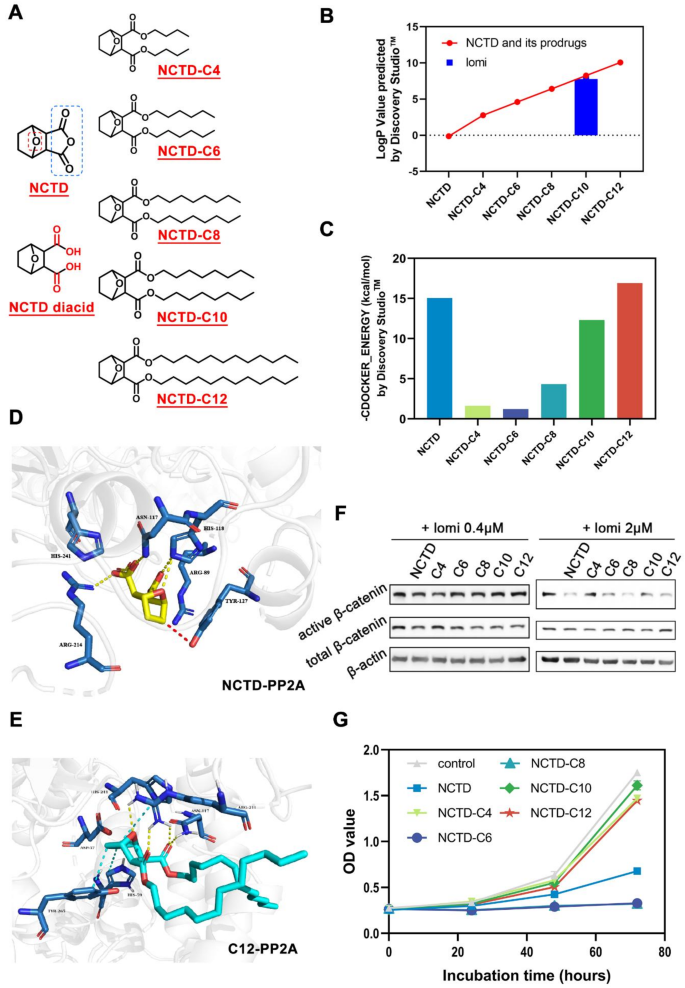
Despite the two carboxyl groups in NCTD diacid causing high polarity and leading to fast partition into the aqueous environment from the NP core, they are indispensable for the pharmacological activity of attenuating β-catenin [20, 37]. The esterification of carboxyl functional groups with short or long aliphatic alcohols is one of the most widely used methods in prodrug strategy [38]. Therefore, five NCTD ester prodrugs were designed and synthesized, as indicated in Fig. 3A. In this study, the prodrugs NCTD-C4(C4), NCTD-C6(C6), NCTD-C8(C8), NCTD-C10(C10), and NCTD-C12(C12) were synthesized and characterized by TOF-MS, 1H NMR 13C NMR and Thin-layer chromatography (TLC) (Supplementary Fig. 4–18). The TOF-MS data provided molecular weight and formula information, while the NMR data helped verify the carbon backbone and hydrogen environments. TLC was conducted to assess the purity of the synthesized prodrugs. The results demonstrated clear, distinct spots for each compound, indicating the successful separation of the main components and supporting the purity of the samples. The TLC data for each prodrug are presented in Fig. S19. As predicted by Discovery Studio™ software (Fig. 3B), the lipophilicity (expressed as LogP) of NCTD ester prodrugs was substantially elevated compared to the NCTD lead compound. Among them, the logP values of prodrug NCTD-C8, NCTD-C10 and NCTD-C12 were very close to that of lomi, suggesting their extended-release profiles when encapsulated in NPs with lomi. Cumulatively, the physiochemical properties of the NCTD prodrugs have met our expectations for further investigation.
As PP2A is a key target of NCTD for downregulating β-catenin [18, 19], a further docking study was conducted to investigate the binding modes of the prodrugs to the PP2A protein (Protein Data Bank entry 2IE4). Based on the CDOCKER_ENERGY values, a higher value after docking indicates a more stable combination of the modified compound and the protein. The docking values of NCTD and its prodrugs with PP2A are shown in Fig. 3C. NCTD, as a PP2A inhibitor, has a docking value of 15.0532. Among the synthesized prodrugs, the docking values of C4, C6, and C8 are similar but significantly differ from that of NCTD, suggesting that their combination with PP2A may be unstable. In contrast, the docking values of C10 and C12 are close to that of NCTD, with the C12 value being higher, indicating that the combination of C12 and PP2A may be more stable. C12 adopted binding poses in the active site of the 2IE4 protein similar to those of the natural ligand NCTD (Supplementary Fig. 20, 21). The two carboxyl groups of NCTD can form hydrogen bonds (yellow line) with ARG214, ASN117 and HIS118, and the oxo bridge (red line) of the NCTD can form π-alkyl interactions with TYR127 (Fig. 3D). Similarly, the oxo bridge and two carbonyl groups in C12 can form hydrogen bonds (yellow line) with ARG214 and ASN117, and the cyclohexane ring in C12 can form hydrophobic interactions (cyan line) with TYR265, HIS59 and ARG214 (Fig. 3E). Thus, it can be concluded that C12 fits in the active sites of the PP2A protein similar to NCTD, indicating that the non-hydrolyzed NCTD derivatives also have the potential to downregulate β-catenin in a similar manner to NCTD.
Subsequently, we explored whether these NCTD-lipid prodrugs would achieve the desired pharmacological effects similar to NCTD. WB experiment was performed to identify the prodrugs’ potential to suppress β-catenin in combination with lomi in TNBC cell lines. The WB analysis, conducted in triplicate, revealed that the combinations of “C8 + lomi” and “C12 + lomi” exhibited similar effects to “NCTD + lomi” in reducing both total and active β-catenin levels. The quantitative data from three independent WB experiments were analyzed and presented in the accompanying column charts (Fig. 3F; Supplementary Fig. 22). These data consistently demonstrated that the NCTD modification did not affect its in vitro activity compared to unmodified NCTD.
Meanwhile, cytotoxicity was investigated to exclude non-specific effects against β-catenin (Fig. 3G). Compared to NCTD, prodrug C4, C10 and C12 at the dosage of 40 µM showed very mild effects against 4T1 cell proliferation, indicating that β-catenin downregulation by C12 was not due to non-specific cytotoxic effects. Considering lipophilicity, pharmacological activity, and non-specific cytotoxicity, C12 became the final candidate for NP loading.
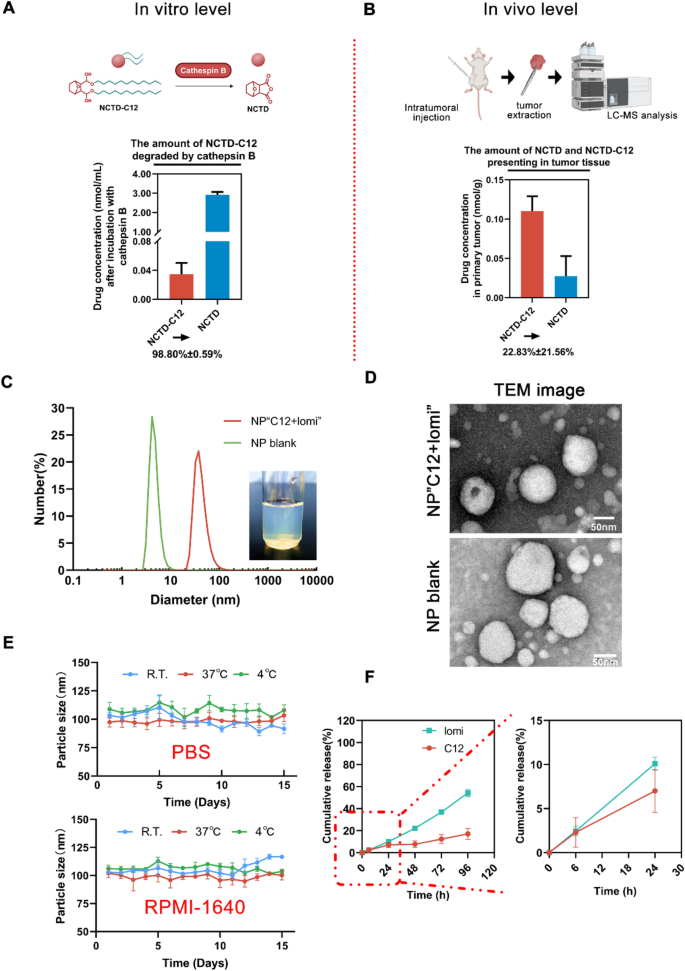
The utilization of a prodrug strategy stands as a promising avenue for optimizing unfavorable physicochemical properties, augmenting stability, and achieving precise delivery without compromising activity. Cathepsins play integral roles in various processes linked to lysosomal functions, including protein degradation, protein and lipid metabolism, autophagy, antigen presentation, recycling of growth factor receptors, cellular stress signaling, and lysosome-mediated cell death [39]. We explored the degradation of released NCTD-C12 in a cathepsin B-sensitive manner via LC-MS/MS analysis. The hydrolysis experiment of NCTD-C12, conducted in the presence of cathepsin B, revealed the transformation of NCTD-C12 into NCTD after 72 h of incubation (Supplementary Fig. 23, 24). In vitro experiments demonstrated that the conversion rates of the prodrug C12 in a solution containing cathepsin B were 98.80% ± 0.59% (Fig. 4A). In vivo experiments (Fig. 4B) showed that in the extracellular environment, only 22.83% ± 21.56% of the prodrug C12 was converted to NCTD, indicating that C12 is more stable in the absence of cathepsin B in vivo. These results suggest that prodrug C12 exhibits high extracellular stability and can be converted to NCTD within cells, thereby prolonging the drug’s therapeutic effect.
Rational Design of NCTD-lipid Prodrugs for Co-Encapsulation with lomi.(A) Molecular structure of NCTD-lipid prodrugs. (B) LogP values of NCTD prodrugs predicted by Discovery Studio™ software, indicating hydrophobic properties. (C) Scoring of the docking of NCTD and its prodrug with PP2A molecules by Discovery Studio™ software. (D) Docking of NCTD (depicted in yellow) into the PP2A (PDB entry 2IE4). (E) Docking of NCTD-C12 (depicted in green) into the PP2A (PDB entry 2IE4). (F) Western blot analysis depicting active β-catenin and total β-catenin levels in TNBC 4T1 cells co-exposed to NCTD prodrugs (5 µM) and lomi (2 µM) for 24 h. (G) Non-specific cytotoxicity analysis of all five NCTD prodrugs via MTT assay. C6 and C8 demonstrated most pronounced effects on TNBC 4T1 cell growth. The data were presented as mean ± SD (n = 6)
Engineering synergism-driving NP“C12 + lomi” formulation
In order to co-encapsulate NCTD-C12 with lomi in a single delivery system, they were mixed with lecithin and DSPE-PEG-RGD (synthesized from MAL-PEG-DSPE and RGD-SH via Michael addition reaction [24]) to form nanoparticles through self-assembly using high-pressure homogenization method. The highly lipophilic NCTD-C12 and lomi formed the hydrophobic core, while the phospholipids of DSPE-PEG-RGD stabilized the nanoparticles via the principle of similar phase solubilization. The hydrophilic terminus of PEG, which is connected to the RGD peptide, enabled tumor-targeting, leading to the successful formation of the NP“C12 + lomi”. The physical properties of these NPs were subsequently characterized. The encapsulation efficiencies (EE%) of C12 and lomi within NP“C12 + lomi” were determined to be 98.22 ± 3.20% and 86.77 ± 6.34%, respectively. At a concentration of 1 mg mL-1 of C12, the size of the loaded NPs was approximately 100–130 nm, with an average diameter of 127.7 ± 1.8 nm, while the blank nanoparticles had a smaller size of 80.2 ± 2.3 nm. The increase in particle size for the loaded NP suggests that drug encapsulation resulted in a larger nanoparticle, which is commonly observed when drug molecules are incorporated. Furthermore, we repeated the Transmission Electron Microscopy (TEM) imaging to obtain representative images with more nanoparticles (Supplementary Fig. 25, 26). The average size of the loaded NPs was 76.2 ± 19.5 nm, while that of the blank nanoparticles was 67.3 ± 25.8 nm. Although the absolute sizes measured by TEM were smaller than those obtained by Dynamic Light Scattering (DLS), this discrepancy is expected due to differences in the measurement principles. Nevertheless, both methods revealed a clear size increase upon drug loading. Furthermore, the size distribution observed from TEM is consistent with the PDI values measured by DLS (0.371 for NP“C12 + lomi” and 0.331 for NP blank), supporting the relatively narrow size distribution and acceptable homogeneity of the nanoparticles. The Zeta potential of the NP“C12 + lomi” was − 35.1 ± 2.2 mV, significantly more negative than that of the NP blank, which exhibited a Zeta potential of -15.1 ± 2.0 mV. This notable difference in Zeta potential indicates that the drug loading not only increased the particle size but also enhanced the stability of the NPs due to the increased negative surface charge (Fig. 4C, D; Supplementary Fig. 27, 28). The particle size of NP“C12 + lomi” in PBS and RPMI-1640 was maintained at approximately 100 nm over 15 days, suggesting good stability under normal physiological condition (Fig. 4E). The DSC analysis of NP“C12 + lomi” shows that the original crystal characteristic peak of C12 and the peak of lomi disappear. This suggests that the drug may have transformed into an amorphous state within the nanoparticles, indicating successful drug encapsulation (Supplementary Fig. 29). TLC analysis showed that the NP blank exhibited only a spot corresponding to the lipid component, while the C12 and lomi NP showed spots corresponding to their respective drug components. The NP“C12 + lomi” displayed two spots, representing the C12 and the lomi, indicating the successful co-encapsulation of both in the nanoparticles (Supplementary Fig. 30). We further investigated the intracellular localization of Cy5-labeled nanoparticles (Supplementary Fig. 31). The results showed significant co-localization of the nanoparticles with the endoplasmic reticulum, supporting their potential as effective drug delivery carriers capable of functioning in specific cellular regions. Collectively, these findings affirm the successful co-entrapment of C12 and lomi, leveraging their respective lipophilic properties.
The underlying design concept of the synergism-driving NPs underscores the importance of an extended-release profile for the simultaneous colocalization of multiple payloads at specific sites [40]. To this end, a release-profile study was conducted. As depicted in Fig. 4F, approximately 10% of C12 or lomi was released from NP“C12 + lomi” after 24 h of incubation in PBS (1% Tween 20) at pH 7.4. Both C12 and lomi exhibited similar sustained-release patterns (Supplementary Fig. 32, 33). In stark contrast, the parent compound NCTD exhibited rapid release from the NP“C12 + lomi”, with nearly 100% of the drug released within the first 24 h. The data for NP“C12 + lomi” best fit the Korsmeyer-Peppas model. For the C12, the release followed the Korsmeyer-Peppas model (R²=0.962), with an exponent value of n = 0.65, indicating anomalous diffusion, where both diffusion and dissolution contributed to the release. In contrast, lomi followed the Korsmeyer-Peppas model (R²=0.993), with an exponent value of n = 1.02, suggesting erosion-controlled release. These findings support the hypothesis that NP“C12 + lomi” can effectively facilitate small molecule colocalization in complex physiological environments, which is crucial for achieving pharmacological synergism. Additionally, we found that the conversion of C12 to NCTD in NPs in the presence of cathepsin B in vitro was 87.52%±5.17% (Supplementary Fig. 34), which differed little from the prodrug C12 conversion efficiency (98.80%±0.59%) mentioned above. The results indicated that the NPs did not affect the efficiency of the conversion of C12 to NCTD. To confirm the physical interaction and co-localization of the molecules, FRET efficiency was monitored over 8 h. As shown in Fig. S35, the FRET efficiency remained stable from 46.91% (0 min) to 45.05% (8 h), with only a minor decrease of 1.86% over the observation period. These results indicate that the molecular interaction remains stable over time, suggesting a persistent close proximity between the donor and acceptor molecules. The minimal fluctuation in FRET efficiency further suggests that no significant dissociation or structural reorganization occurred within the timeframe of the experiment.
Fabrication and characterization of NPs co-loaded with lomi and C12.(A) In vitro analysis of cathepsin B-responsive prodrug NCTD-C12. In the presence of 10 unit mL− 1 cathepsin B, 1 mg mL− 1 NCTD-C12 was incubated in sodium acetate solution (pH 5.0) with 10 U mL− 1 cathepsin B for 72 hours. The final concentrations of NCTD and NCTD-C12 were determined by LC-MS/MS, the transition pairs of NCTD and NCTD-C12 were m/z 168.94→67 and 523.4→123, respectively. (B) In vivo analysis of cathepsin B-responsive NCTD-C12. C12 was administered via intratumoral injection at a dosage of 10 mg kg− 1 when the tumor size reached ≈ 100 mm3. After 72 h, tumors were extracted, homogenized, and analyzed further by LC-MS/MS. (C) Determination of NP“C12 + lomi” size and NP blank using DLS method and the appearance of the NP“C12 + lomi”. (D) Representative TEM image showcasing NP“C12 + lomi” and NP blank. Scale bar, 50 nm. (E) Stability of NP“C12 + lomi” under various conditions. The NP were tested in cell culture fluids (RPMI-1640) and PBS at different temperatures (room temperature, 4 °C, and 37 °C). The particle size was measured over time to assess the stability of the NP under these conditions. (F) In vitro release profiles of lomi, and C12 from NP“C12 + lomi” in pH 7.4 PBS with 1% Tween 20
Enhanced in vitro CCL4 expression mediated by synergistic NP“C12 + lomi”
Inappropriate activation of the Wnt pathway has been shown to drive cell proliferation [30], and β-catenin translocation into the nucleus can result in the activation of multiple genes, including cyclin D1 and cMyc, whose transcription triggers cell proliferation advancement. In order to better demonstrate the advantages of the C12, we conducted a comparative study of nanoparticles co-loaded with various prodrugs and lomi. WB experiments showed that the NP“C12 + lomi” significantly reduced the expression of both total and active β-catenin (Supplementary Fig. 36). Additionally, the CCL4 and CD24/CD44 expression levels in the NP“C12 + lomi” group were significantly higher compared to the other groups (Supplementary Fig. 37, 38). This suggests that the NP“C12 + lomi” formulation not only impacts the β-catenin signaling pathway but may also promote a more favorable immune microenvironment, potentially enhancing the therapeutic effects by altering the stem-like characteristics of the tumor cells. The increased CD24/CD44 ratio indicates a reduction in the cancer stem cell population, which could contribute to the improved anti-tumor activity observed with this formulation. The drug-loaded NPs with varying NCTD prodrugs demonstrated a potent dose-dependent suppression of 3D soft agar colony formation in 4T1 cells (Supplementary Fig. 39). Notably, NPs conjugated with lomi and longer carbon chains (NP“C10 + lomi” and NP“C12 + lomi”) exhibited near-complete inhibition of colony formation, which was significantly lower than both the control group and short-chain counterparts. This trend was further supported by spheroid formation analysis (Supplementary Fig. 40). Treatment with NP“C12 + lomi” resulted in both the smallest spheroid size and the fewest spheroids, outperforming all other groups. The results of the above experiment provide strong evidence that increased hydrophobicity and enhanced drug retention from longer-chain NPs contribute to improved anti-tumor effects.
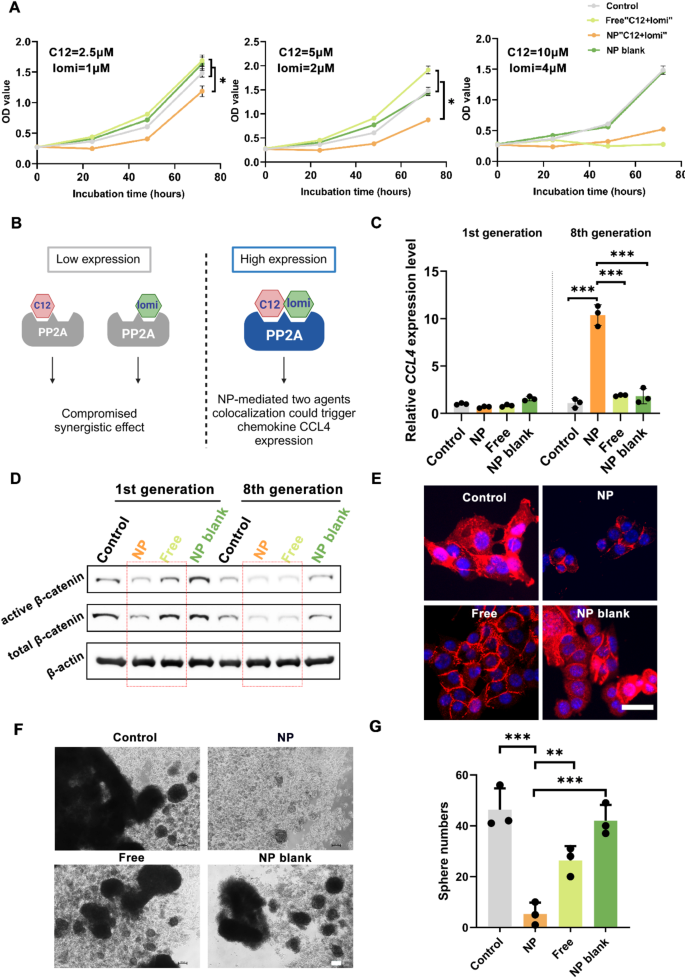
An MTT study (Fig. 5A) was performed to analyze the proliferation of 4T1 cells exposed to NP“C12 + lomi” and free“C12 + lomi”. NP“C12 + lomi” inhibited 4T1 cell growth more efficiently than free“C12 + lomi” at lower dosages (C12 + lomi: 2.5 µM + 1 µM, 5 µM + 2 µM) due to the greater suppression of β-catenin. However, as the dosages increased, the distinction between the two treatments disappeared, which may be attributed to the cytotoxic effects overwhelming the synergistic attenuation of β-catenin. To further assess the synergistic effect of the NP“C12 + lomi” combination, the combination index (CI) was calculated using the formula CI = Cl/IC50 + Cn/IC50, where Cl and Cn represent the concentrations of C12 and lomi. The CI value for NP“C12 + lomi” was found to be 0.11, suggesting a synergistic effect between the two drugs at the tested concentrations. In addition, the hemolysis assay results showed that the positive control group (pure water) caused significant red blood cell lysis, while the negative control group (physiological saline) did not exhibit any hemolysis. The different nanoparticle treatment groups (NP blank, lomi NP, C12 NP, NP“C12 + lomi”) showed similar results to the negative control group, with no significant red blood cell lysis, indicating good biocompatibility of these nanoparticles in blood (Supplementary Fig. 41). We performed a cell migration assay with the same groups at four time points: 0 h, 6 h, 24 h, and 48 h. The final results demonstrated that NP“C12 + lomi” curtailed the migration of TNBC 4T1 cells (Supplementary Fig. 42).
The in vitro cell-based assays explored whether the synergistic NP could efficiently promote CCL4 expression. It was assumed that the integrated NP would achieve the synergism (“high expression”) of “C12 + lomi” more efficiently than the free drugs, which would exhibit reduced efficacy due to NP disassociation (“low expression”) in physiological environments (Fig. 5B). To test this, 4T1 cells were continuously treated with saline, NP“C12 + lomi”, free“C12 + lomi”, and a blank NP vehicle over eight generations. As shown in Fig. 5C, qPCR analysis detected an upward trend in CCL4 expression levels after stimulating 8th generation 4T1 cells with the synergistic NP. Compared to the control group, NP“C12 + lomi” successfully boosted CCL4 expression 10-folds in 8th passage 4T1 cells, whereas the free“C12 + lomi” combination did not alter CCL4 expression. This indicates that long-term treatment with the synergistic NP can induce CCL4 expression in cancer cells.
To determine whether the observed changes were induced by β-catenin suppression, several β-catenin phenotypic studies were performed. As indicated by the WB results in 4T1 cells of 1st culture passage (Fig. 5D; Supplementary Fig. 43), NP“C12 + lomi” attenuated both active and total β-catenin expression levels more effectively than the free“C12 + lomi” treatment, suggesting that the synergistic NP could more efficiently suppress β-catenin by leveraging small-molecule co-localization. As a nuclear-cytoplasmic shuttling protein, the nuclear localization of β-catenin indicates its activation to increase target gene expression [21]. β-catenin immunofluorescence (IF) staining showed that β-catenin is mainly localized in nucleus in 4T1 cells, as evidenced by the merged pinkish color from β-catenin IF staining (red) and nuclear DNA (deoxyribonucleic acid) DAPI fluorescent staining (blue) (Fig. 5E). In line with the WB analysis results showing diminished β-catenin level in 4T1 cells, very little and weak pinkish color was observed in cells exposed to NP“C12 + lomi” or free“C12 + lomi” for 48 h, indicating significantly less β-catenin nuclear localization. Notably, compared to the free“C12 + lomi (5 µM, 2 µM)” group, less β-catenin remained in the cytosol of 4T1 cells in the NP“C12 + lomi (5 µM, 2 µM)” groups.
CSCs are generally enriched in nonattached sphere cultures, reflecting their anchorage-independent self-renewal capacity. To examine this ability, 4T1 cells were cultured under anchorage-independent conditions in serum-free RMPI1640 medium in the absence or presence of C12 and lomi at dosages of 5 µM and 2 µM, respectively. The presence of NP“C12 + lomi” most effectively inhibited sphere formation, as evidenced by the smaller sphere size in Fig. 5F. Fewer spheres also highlighted the potential of the synergistic NP to impair the self-renewal property of CSCs, which is usually maintained by activated Wnt/β-catenin signaling (Fig. 5G). These results were consistent with the qPCR results, suggesting that the synergistic NP likely induced CCL4 expression via β-catenin suppression. Therefore, by leveraging synergism, NP encapsulation safely confers modulator-like functions to the “C12 + lomi” combination and has a great chance to avoid their “on target, off tumor” toxicities in vivo.
In Vitro Assessment of NP“C12 + lomi” for β-catenin deactivation in TNBC 4T1 Cells. (A) Growth curves of 4T1 cells. Cell growth was monitored through optical density (OD) values (n = 6, *P < 0.05). 4T1 cells were treated with the indicated agents for 0, 24, 48, and 72 h. (B) Schematic illustration outlining the synergistic role of NP. This diagram outlines NP synergistic role in toggling the expression of CCL4 high or low. (C) Quantitative PCR analysis of CCL4 expression. 4T1 cells were subjected to various treatments, including saline, NP“C12 + lomi”, free“C12 + lomi”, or NP blank. Each treatment group underwent continuous culture over 8 passages, with a 48-hour treatment duration in each passage (n = 3, ***P < 0.001). (D) Western blot analysis of active β-catenin and total β-catenin levels. 4T1 cells from the 1st and 8th generations were analyzed for active β-catenin and total β-catenin levels. (E) Representative IF images of β-catenin. 4T1 Cells were exposed to saline, NP“C12 + lomi”, free“C12 + lomi”, or NP blank for 48 h and then fixed by 4% paraformaldehyde for further IF staining. Scale bar, 50 μm. (F) Sphere formation assay. This assay demonstrates reduced sphere-forming activity in 4T1 cells with the indicated treatments. The spheres were cultured in a 10-day cycle. Scale bar, 100 μm. (G) Cells were photographed and counted. The graph represents the mean ± SD (n = 3). **P < 0.01; ***P < 0.001
Immunotherapy responses arising from the treatment of synergistic NP
Having confirmed the in vitro potency of the synergistic NP, we next sought to identify its in vivo potency to stimulate immune system. The results of organ distribution of Cy7.5-labeled NPs were showed as Fig. S44. In the NP“C12 + lomi” group, prominent fluorescence signals were observed in both the liver and tumor tissues. Notably, the fluorescence intensity in the tumor region was significantly higher than in other areas, indicating that the nanoparticles effectively accumulated in the tumor. In contrast, free Cy7.5 primarily accumulated in the liver, with relatively weak fluorescence signals observed in the tumor region. This differential distribution suggests that the nanoparticles, through specific biodistribution mechanisms, are able to target and accumulate in the tumor.
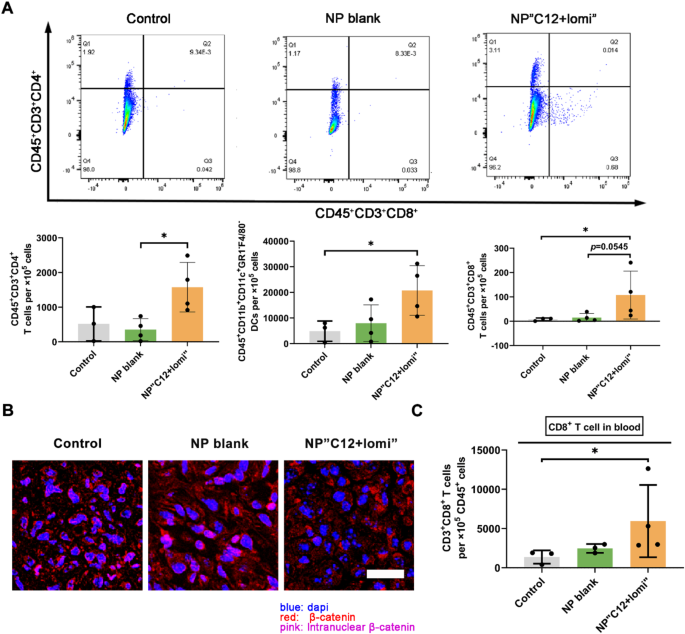
TNBC generally presents as a “cold tumor” with limited effector T cells infiltration, largely attributed to the loss of CCL4 expression in TME [1]. Therefore, we aimed to investigate whether normalizing CCL4 by the synergistic NP could restore immune status and boost immunotherapy for TNBC. A murine TNBC model using 4T1 cells was established to assess this hypothesis. Murine 4T1 cells were orthotopically inoculated into the fourth fat pad of female Balb/c mice. The formulations were administered intravenously (i.v.) twice per week for three weeks once the tumors grew to approximately 100 mm3. As shown in Fig. 6A, fluorescence-activated cell sorting (FACS) analysis of the 4T1 primary tumors revealed increased immune cell infiltration, particularly CD45+CD3+CD8+T cells. Additionally, more CD45+CD11b+CD11C+Gr1–F4/80– dendritic cells (DCs) were infiltrated in the tumor, which typically promote T cell migration to the TME. Thus, the activated CD8+ cytotoxic T-cells returned to the primary tumor site with the help of NP“C12 + lomi”, likely due to the high expression of CCL4. Notably, these therapeutic outcomes were achieved without significant changes in animal body weight, indicating the absence of systemic toxicity (Supplementary Fig. 45).
The accumulation of gene mutations blunts antitumor immunity by excluding CD8+ T cells from tumors in a Wnt/β-catenin signaling-dependent manner [41]. Consistent with this report, we found fewer active β-catenin positive cells in 4T1 primary tumors, as evidenced by the merged pinkish color from β-catenin IF staining (red) and nuclear DNA DAPI fluorescent staining (blue) (Fig. 6B).
Research on CD8+ T cell-dependent antitumor immunity has traditionally emphasized its role within primary tumors. Despite limited impact on primary tumor growth (Supplementary Fig. 46), the treatment group receiving synergistic NPs demonstrated elevated levels of CD8+ T cells in the bloodstream (Fig. 6C), prompting us to further investigate the potential antitumor activity of NP“C12 + lomi”. The increase in cytotoxic CD8+ T cell population in blood suggested NP“C12 + lomi” potential against metastasis.
To further elucidate the potential mechanism of the NP“C12 + lomi” in anti-tumor therapy, we investigated the therapeutic role of either C12 or lomi nanoparticles alone, or NP“C12 + lomi”. We observed the NP“C12 + lomi” significantly increased the number of cytotoxic IFN-γ+ CD8+ T cells, granzyme B+ CD8+ T cells and cytotoxic IFN-γ+ CD4+ T cells in both the tumor tissues and lymph nodes (Supplementary Fig. 47). In addition, the tumor growth curves (Supplementary Fig. 48) showed that the co-loaded nanoparticles significantly suppressed tumor growth compared to the control and single drug treatments. This suggests that the combined therapy of C12 and lomi may promote a more robust activation of CD8+ T cells, potentially enhancing their anti-tumor effects. From the images of the H&E-stained tissue sections (Supplementary Fig. 49), no obvious pathological variation in all groups was observed, indicating that the NP“C12 + lomi” therapy did not induce noticeable tissue toxicity or injury in the studied organs.
NP“C12 + lomi” enhancing cytotoxic CD8+T cells and its potential for combinatorial use with ICI agent. (A) Flow cytometry analysis of immune cell populations in 4T1 primary tumors treated with NP“C12 + lomi”. Flow cytometry was employed to assess immune cell populations, including CD45+CD11b+CD11C+Gr1−F4/80− DC, CD45+CD3+CD4+ T cells, and CD45+CD3+CD8+ T cells, within 4T1 primary tumors treated with NP“C12 + lomi”. Mice bearing 4T1 tumors were euthanized on day 29 post-inoculation, and primary tumors were harvested for analysis, using the gating method detailed in Supplementary Fig. 50. Data with significance were denoted as *P < 0.05. (B) IF staining of β-catenin in 4T1 primary tumors. IF staining was conducted to visualize active β-catenin levels and cellular localization within 4T1 primary tumors. Fluorescent signals depict the relative levels of active β-catenin. NP“C12 + lomi” makes more cytosolic β-catenin undergo the destruction process into the proteasome and less β-catenin were transcribed to suppress CCL4 expression. Scale bar, 25 μm. (C) Comparison of final primary tumor weights and cytotoxic CD8+ T cells in blood among indicated groups. Despite limited impact on primary tumor growth, the increase in cytotoxic CD8+ T cell population in blood suggested NP“C12 + lomi” potential against metastasis
NP-Mediated colocalization of small molecules enhances mitigation of lung metastasis in a post-surgery TNBC model
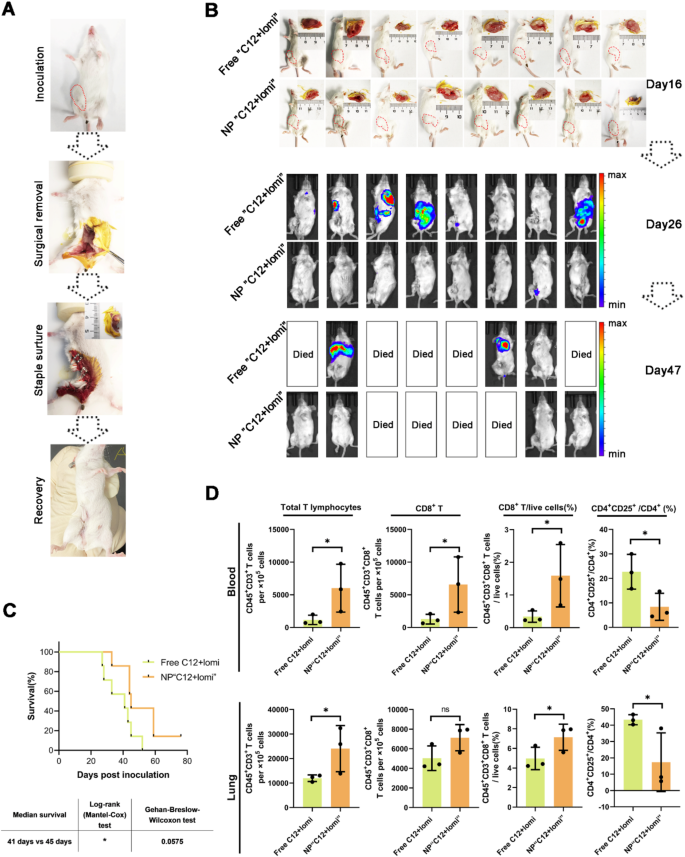
In the landscape of cancer treatments, surgery plays a fundamental role. However, it often faces the risk of incomplete resection and susceptibility to recurrence. To address this issue, we aimed to delineate the primary tumor’s anatomical boundaries and ascertain the post-surgical potential of synergistic NPs in preventing tumor relapse and suppressing metastatic tendencies. To establish an orthotopic breast tumor model characterized by spontaneous metastases, luciferase-expressing 4T1 tumor cells (4T1-Luc) were inoculated into the mammary fat pad of BALB/c mice. When the tumor volume in the mice reached approximately 500 mm3, simulating post-surgery residual micro-tumor conditions, we executed tumor resection. As illustrated in Fig. 7A, this surgical procedure was followed by subsequent recovery. The mice were then stratified into distinct subgroups, each receiving weekly administration of either free“C12 + lomi” or NP“C12 + lomi” with a 7-day interval following tumor resection. We comprehensively evaluated postoperative recurrence and metastasis in TNBC, involving sequential luminescence imaging at relevant junctures or thoracic areas (Fig. 7B). Analytical insights gathered on day 26 and day 47 post-tumor dissection distinctly demonstrated the superior inhibitory efficacy of synergistic NP against tumor metastasis.
Our study highlights the potential of nanoparticle-based treatment as a novel approach to address these challenges. The primary aim of these interventions was to enhance murine survival rates. To this end, a comprehensive survival analysis was conducted, involving meticulous observation of the survival duration of mice over a period of 76 days. In this experiment, day 0 is defined as the day of tumor inoculation. This translated to a substantial improvement in survival rates, as depicted in Fig. 7C. The median survival times for the NP“C12 + lomi” and the free“C12 + lomi” groups were 59 days and 43 days, respectively. The prolongation of the survival period may be attributed to the anti-lung metastasis efficacy of the NP treatment. Additionally, we recorded the longitudinal alteration in body weight, as depicted in Fig. S51. The body weights of mice in each specific treatment group were tracked at 7-day intervals. Crucially, over the entire treatment duration, the body weight of mice in both experimental groups remained remarkably stable, emphasizing the safety profile of the developed synergistic NP throughout the therapeutic regimen. In comparison to traditional postoperative chemotherapy, which often comes with significant side effects and variable efficacy in TNBC, our NP treatment demonstrated several advantages. The targeted delivery of drugs via nanoparticles allows for more precise drug delivery to the tumor site, reducing damage to healthy tissues and minimizing side effects [42]. Additionally, the ability to co-deliver multiple therapeutic agents within a single nanoparticle system enhances the synergistic effects of the drugs, potentially overcoming limitations seen with free drug administration [43].
The distinct therapeutic outcomes of “C12 + lomi” with or without NP suggest an involvement of the immune system. Systemic treatment with STING agonists in mice has been reported to eliminate dormant metastasis and prevent spontaneous outbreaks in a T cell-dependent manner [44]. Thus, we investigated whether the inhibition of tumor metastasis by synergistic NP relies on a T cell-dependent mechanism. FACS analyses of mice lungs and blood revealed increased expression levels of CD8+ T cells in the lungs of NP-treated mice. Significantly increased CD45+CD3+ T cell infiltration was observed in tumors treated with NP“C12 + lomi”. The CD8+ cell populations were also elevated, while the percentage of CD4+CD25+ T regulatory cells decreased (Fig. 7D). Elevated levels of serum CCL4 have been reported to improved disease-free survival [31]. In line with this, the administration of NPs facilitated prolonged inhibition of 4T1 metastasis without inducing significant toxicity. Mechanistically, we observed robust infiltration of immune effector cells, notably CD8+ T cells, into lung tissues and blood. Therefore, the physical co-localization of the two drugs appears to achieve a better therapeutic effect compared to the free drug. Our findings suggest that nanosystems could serve as ideal dual-drug delivery systems, enhancing the synergistic effects between drugs. Subsequent investigations will integrate NP with other immunotherapeutic approaches to assess its synergistic efficacy in suppressing metastatic progression.
Antimetastatic efficacy of NP“C12 + lomi” in the 4T1 post-surgery model due to the colocalization of small molecules. (A) A schematic summary of how the 4T1 post-surgery model was established. (B) Metastases progression was monitored quantitatively in the free“C12 + lomi” and NP“C12 + lomi”-treated groups at day 26 and day 47 after inoculation using biophotonic imaging analysis. (C) Survival analysis of treated mice (n = 7). (D) Quantification of metastases-infiltrating lymphocytes in blood and lung from BALB/c mice (n = 3). *P < 0.05
Synergistic antitumor and antimetastatic effects of application NP“C12 + lomi” with BMS1016
BMS company once designed a series of small-molecule PD-1/PD-L1 interaction inhibitors, such as BMS1016, which significantly increased anti-PD-1/PD-L1 activity [45, 46]. We previously tested the efficacy of BMS1016 combined with a β-catenin suppressor [46] and observed a trend of decreased CCL4 expression was in long-term experiments with the BMS1016, while NP“C12 + lomi” treatment resulted in increased CCL4 expression (Supplementary Fig. 52). Reportedly, combined treatment with PD-1 blockade and Wnt/β-catenin signaling inhibitors induces better antitumor immunity than either treatment alone [41]. Thus, we propose a mechanism-oriented combination therapy where an ICI agent is combined with NP“C12 + lomi”. Similar to our previous study [46], BMS1016 was chosen as the ICI agent due to its intrinsic low immunogenicity as a small molecule.
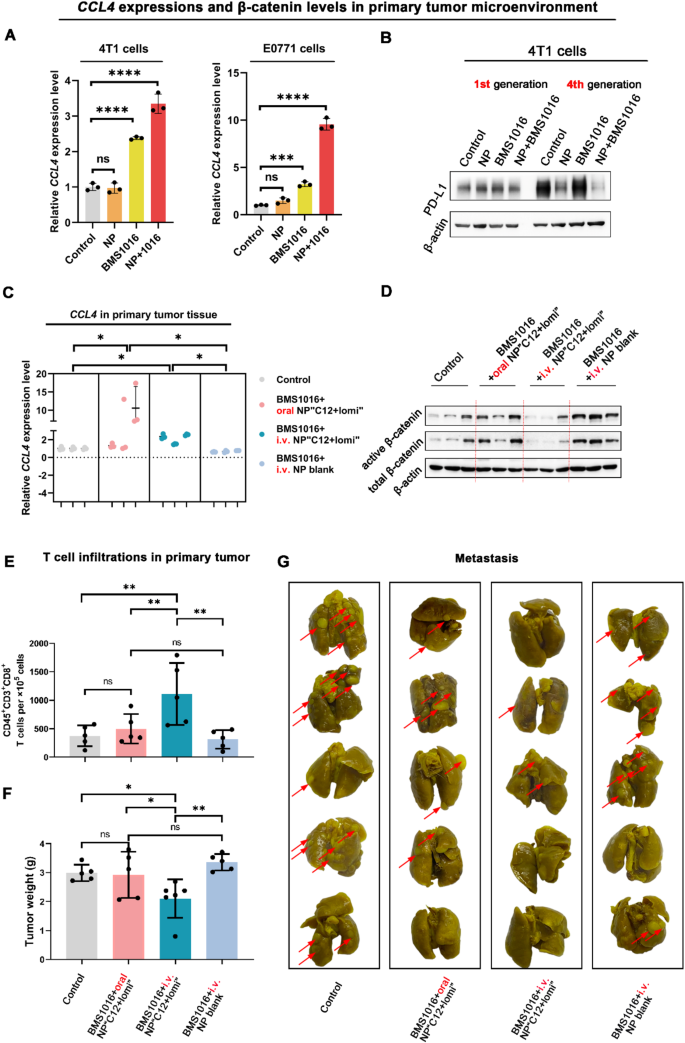
In both 4T1 and E0771 cells, the combinatorial use of NP“C12 + lomi” and BMS1016 significantly elevated the secretion of CCL4 in a synergistical manner (Fig. 8A). Additionally, β-catenin activation can elevate PD-L1 expression in tumor cells by binding the β-catenin/TCF/LEF complex to the CD274 gene promoter region, leading to immune evasion in glioblastoma [47]. In the present study, the combinatorial use of NP“C12 + lomi” and BMS1016 effectively diminished these unfavorable outcomes in 4th passage 4T1 cells. The WB analysis, revealed that the NP“C12 + lomi” + BMS1016 combination treatment significantly reduced PD-L1 expression than the NP“C12 + lomi” or BMS1016. The quantitative results are presented in the accompanying column chart (Fig. 8B, Supplementary Fig. 53). In a tumor cell-T cell co-culture system, the BMS1016 + NP“C12 + lomi” combination demonstrated synergistic immunostimulatory effects, with significantly enhanced T cell proliferation compared to individual treatments (Supplementary Fig. 54), indicating enhanced T cell proliferation and potential immunostimulatory effects of the combined regimen. Taken together, these in vitro results strongly suggest the substantial potential of combining NP“C12 + lomi” with ICI agents and promote further in vivo studies.
We tested the antitumor efficacy of BMS1016 combined with NP“C12 + lomi” in a 4T1 tumor model following the schedule in Supplementary Fig. 55. Small molecules are favored for their better oral availability compared to biologics. Thus, we included an oral administration group in the in vivo study. When tumor volumes reached approximately 100 mm3, groups of five mice received the following treatments: (1)saline, (2)BMS1016 (1 mg kg-1, intraperitoneally, i.p.) + oral NP“C12 + lomi” (20 mg kg-1, 10 mg kg-1)”, (3)BMS1016 (1 mg kg-1, i.p.) + i.v. NP“C12 + lomi” (20 mg kg-1, 10 mg kg-1)”, (4)BMS1016 (1 mg kg-1, i.p.) + i.v. NP vehicle every 3 days for a total of 10 (NP“C12 + lomi”) or 4 (BMS1016) administrations. After 3 weeks of treatment, CCL4 levels in tumor burden were quantified by qPCR (Fig. 8C). Both oral and i.v. NP“C12 + lomi” combined with BMS1016 caused appreciable changes compared to the control mice. We further performed immunoblot analysis of tumor lysates for Wnt markers (total and active β-catenin) to investigate the association between CCL4 expression and β-catenin attenuation in the animals treated with BMS1016 + NP“C12 + lomi”. In agreement with changes in CCL4 expression, we observed appreciable change in total and active β-catenin after BMS1016 + i.v. NP“C12 + lomi” treatments. However, tumors treated with BMS1016 + oral NP“C12 + lomi” did not show the same deactivation, as indicated by the weaker bands, this discrepancy was also reflected in the corresponding quantitative analysis (Fig. 8D; Supplementary Fig. 56).
The moderate efficacy could be explained by the different pharmacokinetic profiles of the oral and i.v. administration routes, evidenced by the lower plasma concentrations in Supplementary Fig. 57. Specifically, i.v. NP“C12 + lomi” administered at the same dosage of 20 mg kg-1 (C12) and 10 mg kg-1 (lomi) resulted in plasma concentrations of C12 and lomi that were over 100-fold higher than those in the oral NP“C12 + lomi” group. This plasma concentration-time profiles upon i.v. administration to rats disclosed the long-circulation feature of i.v. NP. However, even when NP“C12 + lomi” reaches the gastrointestinal tract, it still faces barriers regarding stability and absorption.
In line with the results of CCL4 expression levels, the quantification of CD45+CD3+CD8+ T cell number in primary tumor tissue demonstrated an increase from two treatment modalities: BMS1016 + oral NP“C12 + lomi” and BMS1016 + i.v. NP“C12 + lomi” (Fig. 8E). The more robust contribution came from the BMS1016 + i.v. NP“C12 + lomi” group, which showed a nearly 2-fold increase in CD45+CD3+CD8+ T cells compared to the control group. Moreover, BMS1016 + i.v. NP“C12 + lomi” had the most potent effect on reducing 4T1 primary tumor growth, whereas BMS1016 + NP vehicle had little effect (Fig. 8F). These findings indicate that the combination of intravenously administered NP“C12 + lomi” and immune checkpoint therapy exhibits a pronounced inhibitory effect on orthotopic tumors.
Inappropriate activation of the Wnt pathway has been shown to drive cell proliferation and tumor formation [30]. With most solid-tumor cancers, the biggest danger is not the tumor itself but its ability to metastasize. Metastasis is the leading cause of cancer death [48] and has an inverse association with CD8+ T cells [49]. Activation of the Wnt/β-catenin signaling pathway is observed in various tumor cells and results in metastasis. Significantly, BMS1016 + i.v. NP“C12 + lomi” also showed the most potent anti-metastasis efficacy, evidenced by the reduced lung metastatic nodules in the 4T1 tumor model (Fig. 8G). In contrast, BMS1016 + NP vehicle barely had CD8+ T cell infiltrating and antitumor effects. It is worth noting the sharp outcome distinctions between the oral NP and i.v. NP, which could be explained by the different pharmacokinetic profiles of the administration routes, as evidenced by the lower plasma concentrations in Fig. S57. Overall, these data indicated the potential application of synergistic NP to augment immunotherapy, providing a possible therapeutic strategy for turning cold tumors hot. Oral NP“C12 + lomi” was prone to disassociate in the digestive system due to degradation by stomach acids and proteases, ultimately becoming free small molecules.
Antitumor efficacy of the combinatorial use of BMS1016 and NP“C12 + lomi” in the 4T1 Orthotopic TNBC Model. (A) Enhancement of CCL4 expression in TNBC 4T1 and E0771 cells with combinatorial use. Quantitative PCR analysis demonstrated elevated CCL4 expression in 4T1 and E0771 cells with the combinatorial use of BMS1016 and NP“C12 + lomi”. (B) Reduction of PD-L1 expression in 4T1 cells after long-term combinatorial treatments. WB analysis indicated diminished PD-L1 expression in 4T1 cells following prolonged combinatorial treatments with BMS1016 and NP“C12 + lomi”. (C) qPCR analysis of CCL4 levels in 4T1 primary tumors using GAPDH as an internal control (n = 3, *P < 0.05). (D) WB analysis of 4T1 primary tumors. WB analysis was performed for a panel of Wnt signaling markers, active and total β-catenin with β-actin serving as a loading control. Less β-catenin due to NP“C12 + lomi” treatment was transcribed to suppress CCL4 expression. (E) Flow cytometry analysis of CD45+CD3+CD8+ T cell populations in primary tumors. Flow cytometry quantified CD45+CD3+CD8+ T cell populations in primary tumors collected on day 36 (n = 5, **P < 0.01). (F) Mean primary tumor weights for each treatment group (n = 5; ns, not significant; *P < 0.05; **P < 0.01). (G) Ex vivo images of lungs with metastatic nodules observed after 72-hour immersion in Bouin’s fixative. Tumor metastasis is indicated by the red arrow