Observation and modeling of human RRs structure
In order to biomimetically construct the skin RRs structure, with the approval of the ethics committee and the consent of the volunteers (burn patients), full-thickness skin tissue was obtained to observe and digitally model the structure of the skin RRs. Femtosecond laser pulse microscopic 3D imaging technology, SEM and HE staining are effective methods to observe the structure of skin samples. The 3D imaging results of the skin were shown in Fig. 1A, and the skin RR structure was clear in the BM layer. To obtain native data on the RR structure, skin samples were serially sectioned (6 μm). The results of HE staining (Fig. 1B) and SEM (Fig. 1C) and parameters of RRs structure (Fig. 1E) showed that there were significant differences in the spacing, width and depth of the RR structures at different sites. The scalp presents densely stacked stratum corneum, while the thigh skin and the waist skin present smoother, larger stratum corneum. In addition, we separated the epidermis and dermis layers and observed them by SEM (Fig. 1D). The junction sites between the epidermis and dermis layers had dense collagen structures, and the hemidesmosome structure was obvious. There were honeycomb-like holes in the upper dermis layer of the BM, and basal cells were stably embedded in the ECM through hemidesmosomes. And the hair follicles penetrate the dermis and epidermis layers. Finally, we performed 3D digital modeling of the RRs structure (Fig. 1F and S1) and found that the RR structure was similar to matrix MNs. It has been reported that the shape and density of the RRs change with age, mature in adulthood, and then gradually shrink [21, 22]. Therefore, in this study, we selected the RR parameters of the 21-year-old waist skin to simulate the biomimetic RR matrix MNs.
Native parameters of RR structure of human skin. (A) Laser femtosecond 3D imaging of different skin samples. (B) HE staining of different skin samples. (C) SEM results of different skin samples. (D) SEM results of the contact site of waist epidermis and dermis. (E) Parameters of RRs structure at different sites, including spacing, width and depth. (F) 3D digital modeling of the RRs structure of waist skin
Preparation and physical properties of biomimetic RR microneedles
Gel and HA are natural bioactive components of ECM, with excellent biocompatibility, and have good application prospects in tissue engineering, cosmetology and other fields [23]. We have also found that hydrogels prepared by GelMA and HAMA exhibit excellent biomechanical properties in tissue adhesion, wound healing, and hemostasis [24]. In this study, Gel and HA were used to synthesize GelMA and HAMA respectively to prepare bionic RR MNs. In order to quickly prepare the imitation skin RR MNs, the MN mold was prepared by PDMS, and then the MN patch was formed by solution pouring. The bottom diameter of the formed MNs was 150 µm, the height was 200 µm, and the MN spacing was 200 µm (Fig. 2A). Then we researched the effect of HAMA and GelMA mixed at different final mass ratios (5%HAMA + 3%GelMA, 5H-3G; 5%HAMA + 5%GelMA, 5H-5G; 5%HAMA + 7%GelMA, 5H-7G; 5%HAMA, 5H) and crosslinking time (25, 35, 45, 55, 65, 75 s) on microneedle molding. As shown in Figure S2E, MNs photo-crosslinked at 25 and 35 s could not be stably formed, and the morphology of MNs was intact at 45, 55, 65 and 75 s. Therefore, these crosslinking times of 45, 55, 65 and 75 s were selected to continue studying the physical properties of biomimetic MNs. For further characterization of MNs, we analyzed the elastic modulus (G’) and the viscous modulus (G’’) as a function of angular frequency (ω), oscillation time, oscillation strain and flow frequency at 25 ºC. In the oscillation time and frequency sweep curves (Fig. 2B-C, S2A-B), progressive enhancement in G’ and G” was observed with prolonged crosslinking durations and elevated GelMA concentrations, exhibiting a dominant elastic solid behavior (G’ > G”), which supports long-term cellular encapsulation. In the amplitude scanning analysis (Fig. 2D, S2C), a narrow linear viscoelastic region (strain ≤ 10%) was found in all groups, indicating a brittle hydrogel. Notably, GelMA incorporation improved network resilience through secondary covalent interactions. Flow sweep curves (Fig. 2E, S2D) demonstrated that hydrogels photo-crosslinked at 45 and 55s maintained shear stresses below 200 Pa under physiological shear rates (0.1–10 s⁻¹), ensuring encapsulated cells could potentially be protected from mechanical damage. Given that the C-M in this study was intended to be used for granulation tissue after large skin defects, the mechanical strength requirements for MN were relatively low, and we only needed to ensure that the cross-linking time was sufficient for the efficacy and functionality of cell delivery. Therefore, we selected 45 and 55 s as the optimal cross-linking times for subsequent studies. SEM observation of the surface morphology of the MNs matrix (Fig. 2F) found that the surface of the MNs of 5H-3G, 5H-5G, and 5H-7G after cross-linking for 45 and 55 s was smooth and had dense and interconnected porous structures. ImageJ counted the pore sizes of each group (Fig. 2G), and the 5 H had a larger aperture structure. In addition, the moisture balance of the wound will affect wound healing, so the water-absorbing and water-retaining properties of the MNs were further tested. The swelling performance results of MNs showed that at 45 and 55 s (Fig. 2H), the swelling rate of 5% HAMA alone was the highest, and the lowest in the 5H-7G group. There was no difference in the swelling ratio (55 s) among all groups compared with that at 45 s. For the water-retaining capacity of MNs (Fig. 2K and S2F), in all groups photo-crosslinked for 45 s, the water retention capacity was lower compared with that at 55 s. Finally, the MNs were treated with 2.5 U/ml collagenase type II solution in vitro to simulate the in vivo degradation rate (Fig. 2I and J and S2G), and the degradation rates of 5H-3G, 5H-5G, 5H-7G and 5H after gelation within 55 s were slower than those after gelation within 45 s. In summary, the MNs formed by 5H-5G at 55 s of photo-crosslinking have complete moldability, appropriate pore size, storage modulus and degradation speed, and have good swelling ratio and water retention properties. Therefore, the bionic RR MNs formed by 5H-5G at an optical crosslinking time of 55 s were adopted in the subsequent study.
Evaluated the physical properties of MNs. (A) Preparation process and size of MNs. (B) Oscillation time sweep curves related to 5H-5G. (C) Oscillation frequency sweep curves related to 5H-5G. (D) Oscillation strain sweep curves related to 5H-5G. (E) Flow frequency sweep curves related to 5H-5G. (F) SEM results of MNs at 45 and 55 s. (G) Statistical distribution of MN gap size at 45 and 55 s. (H) Swelling properties of cross-linked time of 45 and 55 s MNs after 24 h. (I and J) Degradation of cross-linked time of 55 s MNs in type II collagenase solution in vitro. (K) Water retention properties of MNs at 55 s
Biosafety of bionic skin RR MNs
Biomimetic RR MNs need to have good biological safety for treating wounds. First, we prepared 5H, 5H-3G, 5H-5G, and 5H-7G into extracts in DMEM medium containing 10% FBS, and then evaluated the toxicity of the extracts on mouse 3T3 fibroblasts. The results of living cell staining (Fig. 3A) and CCK8 (Fig. 3B) test showed that there was no significant difference in the number of living cells in each extract group compared with the control at 72 h, and the cell activity was good. Then 1 cm by 1 cm matrix MNs (5H-5G) of approximately 120 mg were transplanted subcutaneously under the skin of the back of C57 mice to evaluate in vivo degradation rate and inflammatory infiltration. By 42 days, the degradation rate of MNs was approximately 60% (Fig. 3D). Compared with the control group, the inflammatory infiltration of the subcutaneous transplantation site of MNs was not obvious and the inflammatory factors (IL-1β, TNF-α) basically returned to the normal levels (Fig. S3A-B), and there was no pathological injury of the organs (heart, liver, spleen, lung, kidney) in the two groups (Fig. 3C). In addition, no hemolysis occurred in the microneedle extract (Fig. S3C).
The RR MNs designed in this study need to deliver EpiSCs, so we further evaluated the ability of the RR MNs to load EpiSCs. C-Ms were prepared by mixing 1.6 × 10^6 EpiSCs with 1 ml MN solution, and were cultured conventionally. After 24 h, the survival of EpiSCs was observed by living cell staining, the expression of EpiSCs specific markers (CD71 and CD49f) was detected by flow cytometry, and the expression of representative genes of EpiSCs proliferation, senescence and tumorigenesis was detected by qPCR. As shown in the staining results of living cells (Fig. 3E), living cells were present at the bottom, middle and top of the MNs. The proportion of living cells remained above 90% after 24 h (Fig. S3D). There was no statistical difference in the proportions of CD71− and CD49f+ between the C-Ms and control (Fig. 3F). The effects of MNs on EpiSCs gene expression showed that compared with control (Fig. 3G), the expressions of P53 and CDK4 in MN group was increased (P < 0.05), while the expressions of PRB were decreased (P < 0.05), and the expressions of P16, Kras and MYC were not significantly different between the two groups. The above results indicate that C-Ms have good biosafety and can be used for further studies.
Evaluated the biosafety of bionic RR MNs. (A) Live cell staining of 3T3 cells treated by RRs extract. (B) CCK8 results of 3T3 cells treated by RRs extract. (C) HE staining results of the heart, liver, spleen, lung, kidney. (D) Degradation of MNs at different time points after subcutaneous transplantation. (E) At 24 h, the survival of EpiSCs in MNs was observed using a confocal laser microscope. (F) At 24 h, the expression of CD71−CD49f+ in EpiSCs in C-Ms and the control group was detected by flow cytometry. (G) The expression of representative genes of EpiSCs was detected by qPCR. (*P < 0.05)
C-Ms accelerate wound healing in mice through rapid vascularization and promote skin RRs regeneration
To observe the survival of EpiSCs after biomimetic EpiSC-microneedles (C-Ms) implantation in the wound, we labeled EpiSCs with Dil and prepared them into C-Ms. And then, C-M was transplanted into a 1 cm by 1 cm full-thickness skin wound in nude mice and was followed up for 21 days (Fig. 4A). Wound samples were collected for cryosections on postoperative day 7 and day 21. Laser confocal results showed that implanted EpiSCs were observed in the BM layer of the skin, but the number of surviving EpiSCs decreased by day 21. After that, C-Ms without Dil label were used for follow-up experiments. Controls included an untreated wound, wounds treated with only cells and MNs respectively. Wound closure in each group was recorded by digital camera (Fig. 4B), and wound unclosed area was measured by planimetry (Fig. 4C and D). By day 7, the wound unclosed area in the MNs, cell and C-Ms groups was significantly larger than that in the Ctrl (P < 0.001), and there was no significant difference among the MNs, cell and C-M groups. By day 14, the wounds of in the cell, MNs and C-M group was completely closed. By day 21, the wound in the Ctrl was completely closed. HE staining at day 7 and day 21 confirmed the closure efficiency of C-M treated wounds (Fig. 4E and G). By day 7, the un-epithelialization in the MNs, cell and C-Ms groups was significantly greater than that in the control group (Fig. S4A, P < 0.001). By day 21, wound epithelialization was completed in each group, but the epidermal barrier function of the wound in the C-M treatment group was the most obvious, and the new epidermis was thicker and had a multi-layer epidermal structure, which was close to that of normal skin (Fig. S4B).
Early neovascularization provides necessary nutrients for wound healing, accelerates wound repair and inhibits scar formation, so vascularization is a key factor in evaluating skin wound healing [25]. Capillaries were immune-stained with CD31 to quantify vascularity of the healing wounds on days 7 and 14. By day 7 (Fig. 4F and S4D), C-M treated wounds showed higher blood vessel density (67.3 ± 4.1) compared to the Ctrl, cell and MN groups (25.3 ± 2.1, 49.3 ± 4.9, 33.3 ± 3.3; P < 0.01). By day 14 (Fig. S4C and S4E), after the wound was completely healed, the blood vessel density of the C-M treatment group (19.3 ± 2.8) gradually returned to normal levels compared with the Ctrl, cell and MN groups (46.3 ± 4.4, 33.0 ± 3.7, 27.6 ± 2.4; P < 0.01). The C-M treated wounds formed human-like RRs by day 21 (Fig. 4G), and the MN group alone also had part of RRs formation, while the epidermis of the Ctrl and cell groups was flat and had no RRs formation.
C-Ms accelerate wound healing, rapid vascularization, and promote skin RRs regeneration. (A) Living cells tracked the survival of transplanted EpiSCs at different times after C-Ms were transplanted to the wound. EpiSCs were marked with Dil dye. The diameter of the full-thickness skin defect wound in nude mice was 1 cm. (B) Digital camera photographed the wounds in each group at different time points. The inner diameter of the blue circle was 1 cm. (C and D) Statistical results of unclosed wound area in each group at different time points. (E) HE staining results on the 7th day of each group. The blue line, the red box, and the red arrow indicated the location of epithelialization. (F) On day 7, showed Alexa Fluor® 555 anti-mouse CD31 immunostaining results of wounds in each group. White boxes and white arrows represented new blood vessels. (G) On day 21, HE staining results in each group. The red arrow indicated human-like new RRs
C-Ms promotes skin RRs formation and provides niche for episcs
In order to elucidate the mechanism of C-Ms promoting the regeneration of human-like RRs structure on the wound of nude mice, healed wound samples were collected 14 days after surgery for RNA sequencing analysis. Studies have reported that mechanical MN molds are used to simulate RRs in vitro and then transplanted into wounds to reconstruct RRs with poor fidelity [26]. Therefore, we further focused on the influence of MN on RRs formation. Differential gene statistics were shown below (Fig. 5A), compared with the Ctrl group, there were 3054 up-regulated genes and 2305 down-regulated genes in the MN group; and there were 2614 up-regulated genes and 1792 down-regulated genes in the C-M group. However, there was no significant change in the number of differential genes in the MN group compared with the C-M group (Fig. S5A). Using Wayne diagram displaying of the differential genes in the Ctrl, MN and C-M groups, we found that 1697 differential genes (gene set 1) were only in the MN vs. the Ctrl, 744 (gene set 2) were only in the C-M vs. the Ctrl, and 3662 (gene set 3) were in the intersection (Fig. S5B). Then, KEGG enrichment analysis of the gene set 3 found that focal adhesion kinase (FAK), ECM receptor interaction, and other pathways were significantly enriched, which were related to the differential genes LAMA4, ITGB1 and FN1 (Fig. 5B and S5C and S5D). ECM provides anchoring sites for basal cells. Upon binding to ECM components such as laminin and collagen via integrins, FAK is activated, triggering downstream signaling pathways that regulate the polarization and migration of basal cells, thereby promoting the morphogenesis of the reticulate ridge [27, 28]. qPCR analysis of ITGB1, FAK1, COL1A1, and LAMA4 revealed significant activation of ECM receptor interaction and FAK in the C-Ms group (Fig. 5F and S5F). Moreover, LAMA4 is the main regulatory molecules involved in the formation of BMs [29]. Therefore, we performed immunofluorescent staining of LAMA4 on the wounds 14 and 21 days after surgery. On day 14 (Fig. S6) and day 21 (Fig. 5D and E), the expression of LAMA4 in the C-M treated group was significantly higher than those in the MN and the Ctrl (P < 0.001), and the MN group was significantly higher than the Ctrl (P < 0.05).
Further GO enrichment involved the signaling pathway for the EpiSCs maintenance, and the results revealed characteristic changes related to stem cell proliferation, cell adhesion, and epidermal cell differentiation (Fig. S5E). GSEA analysis showed that Notch, Wnt and ERK-related signaling pathways were significantly affected after C-M treatment compared with MN (Fig. 5C). Studies have reported that the loss of Notch signaling leads to premature differentiation of basal cells, disrupting epidermal stratification and the structure of RRs [30]. Wnt/β-catenin signaling promotes ECM remodeling at the dermal-epidermal junction by regulating MMP9 expression. The absence of ERK activity results in reduced proliferation of epidermal basal cells and disorganization of RRs structure [31]. Based on qPCR analysis of NOTCH1, Ctnnb1, Axin2, and Ccnd1, we found that Notch, Wnt/β-catenin, and ERK pathways were significantly activated in the C-Ms group (Fig. 5F and S5F). In order to intuitively study stem cell maintenance, we analyzed the expression of epidermal cell proliferation marker Ki67 and stem cell marker ITGB1 in each group. The results showed that Ki67 and ITGB1 were more abundantly expressed in the C-M treatment (Fig. 5D and E). In general, C-M treatment is more conducive to providing a niche for stem cells.
C-Ms promotes skin RRs formation and provides niche for EpiSCs. (A) The volcano map shows up-regulated and down-regulated genes identified 14 days after treatment with MN and treatment with C-M. (FC ≥ 2.0, P < 0.05). n = 3. (B) KEGG analysis of genes significantly changed in the gene set 3. (FC ≥ 2.0, P < 0.05). (C) Gene set enrichment analysis (GSEA) of RNA-seq data for C-M vs. MN. (D) Representative immunofluorescent staining of control, cell only, MN only and C-M only 21 days after transplantation. Top row: Anti-mouse laminin4 (red), DAPI (blue) and anti-CK14 (green). Middle row: Anti-mouse ITGB1 (pink), DAPI (blue), and anti-CK14 (green). Bottom row: Anti-mouse Ki67 (pink), DAPI (blue), and anti-CK14 (green). (E) shows the statistical result of the fluorescence Fig. 5D. (F) The expression of representative genes of skin tissues (day 21) was detected by qPCR. (*P < 0.05)
C-Ms improve wound healing quality in mice by remodeling extracellular matrix
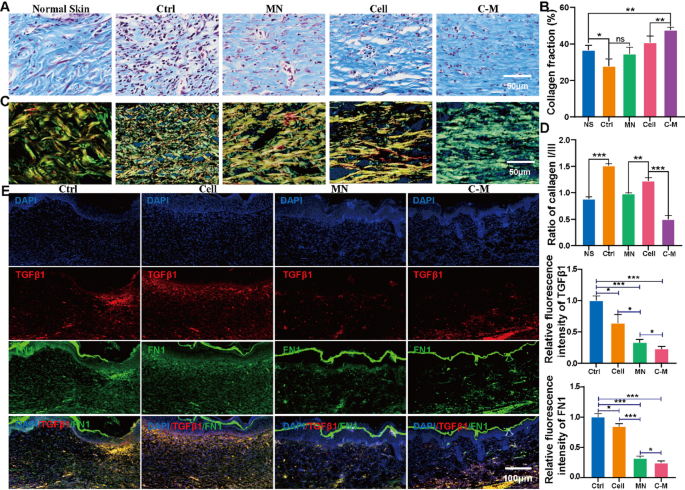
ECM remodeling is important for normal wound healing and reduced fibrosis. Masson staining was used to determine total collagen expression in regenerated skin wounds; Picrosirius red staining further determined the expression of type I and III collagen and collagen architecture. By day 21, Masson results were as follows (Fig. 6A and B); the total collagen fraction in the C-M group (36.7 ± 2.1%) was significantly higher than that in the Ctrl, cell and MN groups (27.9 ± 3.1%, 40.7 ± 2.8%, 34.7 ± 2.8%, respectively; P < 0.01); Picrosirius red results were as follows (Fig. 6C and D); the proportion of type III collagen in the C-M treatment group (0.9 ± 0.0%) was significantly higher than that in the Ctrl, cell and MN groups (1.5 ± 0.0%, 1.2 ± 0.0%, 1.0 ± 0.0%, respectively; P < 0.01). Its blue fibrous tissue was similar to normal skin. In addition, the wound fibers treated in the MN, cell and C-M groups were thick, with parallel fiber orientations and increased fiber thickness, while the fibers in the Ctrl were disorganized and the fibers were smaller.
Our research team previously reported that MNs regulate the process of skin fibrosis through mechanical action and inhibit scar formation [13]. TGFβ1 and FN1 are classic marker genes of fibrosis, so we further measured the expression of TGFβ1 and FN1 in healed wounds. By day 21 (Fig. 6E, F and G), immunofluorescent staining analysis showed that the expression of TGFβ1 in wounds in the C-M treated group was significantly lower than that in the MNs group (P < 0.01). Compared with the group and control group, the expression of TGFβ1 decreased in the MN group (P < 0.01). In addition, the expression of FN1 in each group had a similar trend to that of TGFβ1. The expression trends of TGFβ1 and FN1 mRNA in the skin tissues (day 21) detected by qPCR were consistent with the results of immunofluorescence (Fig. S5F).
C-Ms remodeled the extracellular matrix. (A) Masson’s staining of the C-Ms group, MN group, Cell only group and wound only group at day 21. (B) It was the statistical result of A. (*P < 0.05). (C) Picrosirius red staining of the C-Ms group, MN group, Cell only group and wound only group at day 21. (D) It was the statistical result of collagen I / III of C. (*P < 0.05). (E) Representative immunofluorescent staining of control, cell only, MN only and C-M only 21 days after transplantation. Anti-mouse TGF β1 (red), DAPI (blue) and anti-mouse FN1 (green). (F and G) were the relative fluorescence statistics of TGFβ1 and FN1 of E. (*P < 0.05)