CPP-CDs exhibit significant anti-inflammatory potential
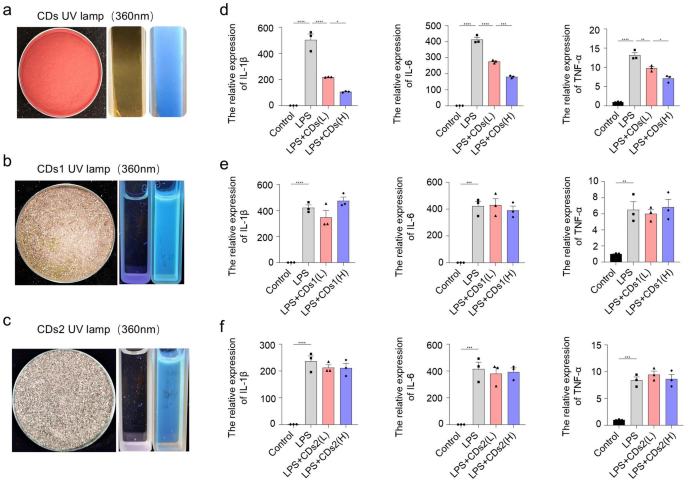
Previous studies have shown that biomass-derived carbon dots not only retain the inherent biological activity of their precursors but also exhibit distinct fluorescence properties, with some even demonstrating enhanced biological efficacy [21]. We investigated the potential functions of purple sweet potato-derived CDs, including CPP-CDs, crude extract of purple sweet potato stems and leaves (CPSPSL-CDs) and purple sweet potato residue (SPR-CDs), in LPS-induced acute inflammation model. All three types of carbon dots exhibited fluorescence (Fig. 1a-c). LPS stimulation significantly increased IL-1β, IL-6, and TNF-α expression. However, only CPP-CDs significantly reduced these genes’ expression in a dose-dependent manner (Fig. 1d-f). Fluorescent digital images show that CPP-CDs maintain stable blue fluorescence at different concentrations (Supplementary Fig. 1a). Additionally, we compared the anti-inflammatory effects of CPP-CDs and CPP, and the results indicate that CPP-CDs exhibit more significant efficacy (Supplementary Fig. 1b).
Effects of three carbon dots on inhibiting LPS-induced inflammation. a-c. Left: Images of the raw material extracts for the three types of carbon dots. Right: Images of the three types of carbon dots under UV light. CDs: CPP-CDs, CDs1:CPSPSL-CDs, CDs2: SPR-CDs. d-f. Relative gene expression levels of IL-1β, IL-6, and TNF-α measured by RT-qPCR. BMDMs were treated with LPS (1 µg/mL, 4 h), low-concentration CPP-CDs (L, 0.5 mg/mL, 8 h), and high-concentration CPP-CDs (H, 1 mg/mL, 8 h) (n = 3 biological replicates). g. Relative gene expression levels of IL-1β, IL-6, and TNF-α measured by RT-qPCR. BMDMs were treated with LPS (1 µg/mL, 4 h), PSPCE and CPP-CDs (both 1 mg/mL, 8 h) (n = 3 biological replicates). d-g. Data were analyzed by One-way ANOVA for multiple group comparisons. *p < 0.05, **p < 0.01, ***p < 0.001
Characterization of CPP-CDs
Characterization of CPP-CDs
The morphology and chemical structure of CPP-CDs were characterized using various techniques. TEM images revealed that CPP-CDs exhibited a uniform spherical structure with particle sizes ranging from 2 to 4 nm, averaging 3.5 nm (Fig. 2a, b). Additionally, SEM images of the precursor CPP showed a complex structure with multiple components and relatively large particle sizes (all larger than 100 nm) (Supplementary Fig. 2). In terms of optical properties, CPP-CDs displayed light absorption in the UV region, extending into the visible light range. The absorption peak at 280 nm was attributed to the π-π⁎C = C transition (Supplementary Fig. 3a). The bandgap energy of CPP-CDs was estimated to be 3.65 eV, classifying them as direct bandgap materials (Supplementary Fig. 3b). Fluorescence spectroscopy revealed that the emission peak of CPP-CDs shifted from 461 nm to 498 nm with different excitation wavelengths between 360 nm and 500 nm (Supplementary Fig. 3c). Under 371 nm excitation, CPP-CDs exhibited a maximum emission peak at 459 nm (Fig. 2c), indicating their excellent PL properties. Using quinine sulfate (0.1 M H2SO4 solvent; quantum yield QY = 54%) as a reference [29], the quantum yield (QY) of CPP-CDs was calculated to be 8.45%. FTIR spectroscopy analysis revealed the chemical structure of CPP-CDs (Fig. 2d). The broad band at 3417 cm⁻¹ corresponded to the stretching vibrations of -OH groups. Additional absorption peaks at 2933 cm⁻¹, 1709 cm⁻¹, 1605 cm⁻¹, 1278 cm⁻¹, and 1201 cm⁻¹ corresponded to the stretching vibrations of C-H, C = O, C = C, C-OH, and C-O groups, respectively. The X-ray diffraction (XRD) pattern showed a broad peak, indicating the amorphous nature of the CDs (Fig. 2e). To further analyze the elemental composition and chemical bonding of CPP-CDs, we performed XPS analysis. The full spectrum revealed that the main elements in CPP-CDs were carbon (C 1s) and oxygen (O 1s) (Fig. 2f). High-resolution XPS C1s spectra showed various bonding states of carbon atoms, including C = C (284.8 eV), C = O (286.4 eV), and O-C = O (289.2 eV) (Fig. 2g). The O1s spectra indicated functional groups such as C-OH (531.89 eV) and C = O (533.07 eV) (Fig. 2h). To further investigate the origin of the functional groups in CPP-CDs, we analyzed the FTIR spectra of CPP and identified its components. The FTIR results showed an absorption peak at 3421 cm⁻¹, which primarily corresponded to the O-H stretching vibration mode, with a higher peak intensity indicating a rich presence of polyphenols, anthocyanins, polysaccharides, and water. The absorption peaks at 2825 cm⁻¹ and 2853 cm⁻¹ in CPP were related to the C-H stretching vibrations of the polysaccharide backbone and lipophilic components. The peak at 1697 cm⁻¹ was attributed to C = O stretching vibrations, primarily derived from the ketone and phenolic acid structures in anthocyanins. Absorption peaks at 1635 cm⁻¹, 1607 cm⁻¹, and 1516 cm⁻¹ were mainly due to aromatic C = C vibrations, involving phenolic and anthocyanin components. The absorption peak near 1277 cm⁻¹ was more complex, mainly related to C-O-C structures. The absorption peak at 1074 cm⁻¹ was primarily attributed to the C-O stretching vibration mode of the glycosidic bond in polysaccharides(Supplementary Fig. 4). These results suggest that the functional groups in CPP-CDs are mainly derived from CPP, and their presence may be linked to the potential anti-inflammatory activity of CPP-CDs. Moreover, the stability of CPP-CDs was evaluated over an extended period, and no significant changes in particle size, zeta potential, or fluorescence intensity were observed, confirming the excellent physical stability of CPP-CDs [30] (Supplementary Fig. 5).
Synthesis and characterization of CPP-CDs. a. TEM images of CPP-CDs showing an overall size of 50 nm and individual particles measuring 5 nm. b. Size distribution analysis of CPP-CDs. c. Fluorescence excitation and emission spectra of CPP-CDs. d. FTIR spectra of CPP-CDs. e. XRD patterns of CPP-CDs. f. Full survey XPS spectra of CPP-CDs. g. High-resolution XPS spectrum of C 1s for CPP-CDs. h. High-resolution XPS spectrum of O 1s for CPP-CDs
CPP-CDs inhibit the inflammatory phenotype induced by LPS in macrophages
The anti-inflammatory effects of CPP-CDs were systematically investigated. ELISA results showed that CPP-CDs treatment significantly reduced IL-1β, IL-6, and TNF-α levels in cell supernatants (Fig. 3a). Inflammation is often accompanied by oxidative stress, characterized by an imbalance of intracellular ATP and ROS levels [31]. ATP assays showed that LPS treatment significantly decreased intracellular ATP and dramatically increased extracellular ATP, however, CPP-CDs treatment restored intracellular ATP and suppressed extracellular ATP elevation (Fig. 3b, c). Meanwhile, LPS treatment significantly increased ROS levels, while CPP-CDs significantly inhibited ROS accumulation (Fig. 3d). These results suggests that CPP-CDs may alleviate inflammation by mitigating oxidative stress. Moreover, LPS treatment induced significant morphological changes in macrophages, such as pyroptotic vesicle formation. Meanwhile, LDH secretion was significantly increased, indicating exacerbated pyroptosis and cell damage. However, CPP-CDs treatment effectively suppressed pyroptosis, as shown by reduced pyroptotic vesicles and decreased LDH secretion(Fig. 3e, f). In addition to their anti-inflammatory and antioxidant effects, CPP-CDs also regulated macrophage polarization. qPCR results showed that CPP-CDs upregulated M2 macrophage markers, ARG1 and CD206 (Fig. 3g), suggesting that CPP-CDs promote macrophage polarization toward the anti-inflammatory M2 phenotype, regulating the intracellular inflammatory response. In summary, in vitro experiments showed that CPP-CDs exhibited significant anti-inflammatory effects in the LPS-induced inflammation model, likely through multiple mechanisms.
Effects of CPP-CDs on LPS-induced inflammation. a. ELISA measurements of IL-1β, IL-6, and TNF-α protein levels in BMDMs culture supernatants. Experimental groups were treated with LPS (1 µg/mL, 4 h), Nigericin (Nig, 5µM, 45 min), and various concentrations of CPP-CDs (n = 4 biological replicates). b-c. ATP levels were measured using an ATP assay kit to assess intracellular and extracellular ATP levels in BMDMs (n = 4 biological replicates). d. ROS levels in BMDMs were measured using a ROS detection probe (n = 3 biological replicates). e. Morphological changes in BMDMs were observed under an optical microscope for each treatment group. Red arrows indicate pyroptotic cells (n = 3 biological replicates). Scale bar: 20 μm. f. LDH release levels in BMDMs for each treatment group, represented as the percentage of LDH activity in the cell lysate (n = 4 biological replicates). g. The relative expression of M2 macrophage markers ARG1 and CD206 was measured by RT-qPCR in BMDMs treated with various concentrations of CPP-CDs (n = 3 biological replicates). a-g. Data are expressed as mean ± standard error of the mean (SEM). One-way or Two-way ANOVA was used for multiple group comparisons. *p < 0.05, **p < 0.01, ***p < 0.001
CPP-CDs mitigate inflammation progression in vivo
An LPS-induced acute inflammation model in mice was established to evaluate the in vivo anti-inflammatory effects of CPP-CDs (Fig. 4a). In a 72-hour survival analysis, all LPS-injected mice died, while CPP-CDs treatment significantly improved survival. In the low-dose group, 50% survived, and in the high-dose group, 67% survived, significantly higher than in the LPS group (Fig. 4b). Histopathological analysis of colon and liver tissues was performed using H&E staining to assess the protective effects of CPP-CDs on tissue damage. In the control group, colonic tissue structure was normal, and glands were arranged orderly. In contrast, the LPS-treated group exhibited significant damage, including disruption of colonic mucosal villi and glandular degeneration. Liver tissue showed disordered hepatocyte arrangement, localized necrosis, and inflammatory cell infiltration around the central vein. However, in the CPP-CDs treatment group, these inflammatory damages were significantly reduced, with more intact colon and liver tissue structures and less inflammatory cell infiltration (Fig. 4c). Quantitative pathological scoring confirmed that CPP-CDs significantly reduced colon and liver damage scores (Fig. 4d). Additionally, LPS treatment significantly increased serum pro-inflammatory factors (IL-1β, IL-6, and TNF-α), while CPP-CDs treatment significantly inhibited their release (Fig. 4e).
In vivo anti-inflammatory effects of CPP-CDs in an LPS-induced mouse model. a. Mouse model construction process: Mice were divided into groups and given intraperitoneal injections of low (L: 15 mg/kg) or high (H: 30 mg/kg) concentrations of CPP-CDs, followed by LPS injection (5 mg/kg) 2 h later. b. Survival rate of mice monitored for 72 h after LPS and CPP-CDs treatment (n = 6, two independent experiments). c. Colon and liver tissues were collected 24 h after LPS and CPP-CDs treatment and stained with H&E to observe tissue damage (magnification: 15×). d. Pathological scoring of colonic lesions and liver inflammation. Colon score was based on weight loss, rectal bleeding, and stool consistency; liver score was based on the number and area of inflammatory foci around the central vein (n = 6, two independent experiments). e. ELISA measurements of IL-1β, IL-6, and TNF-α levels in mouse serum (n = 4 biological replicates). b, d-e. All data are expressed as mean ± standard error of the mean (SEM). One-way or Two-way ANOVA was used for multiple group comparisons. *p < 0.05, **p < 0.01, ***p < 0.001
CPP-CDs inhibit activation of TLR4/NF-κB and NLRP3 pathways in macrophages
The TLR4/NF-κB signaling pathway and NLRP3 inflammasome activation are key steps in LPS-induced inflammation [32, 33]. We further investigated CPP-CDs’ regulatory effects on these pathways. Western blot analysis showed that LPS treatment significantly upregulated TLR4 and p-P65 expression, while CPP-CDs treatment significantly inhibited their expression (Fig. 5a and Supplementary Fig. 6a). Immunofluorescence analysis confirmed the suppression of NF-κB signaling. Compared to the LPS-only group, CPP-CDs treatment significantly reduced p-P65 accumulation in the nucleus, indicating that CPP-CDs inhibit NF-κB nuclear translocation to exert anti-inflammatory effects (Fig. 5b). Western blot results showed that LPS stimulation significantly increased NLRP3 expression, along with caspase-1 and GSDMD cleavage, and increased the secretion of mature IL-1β. However, CPP-CDs treatment significantly inhibited NLRP3 expression, caspase-1 and GSDMD cleavage, and IL-1β secretion. (Fig. 5c and Supplementary Fig. 6b). ASC speck formation assays confirmed that CPP-CDs inhibited NLRP3 inflammasome activation (Fig. 5d), further supporting their anti-inflammatory potential. NF-κB activation in inflammation is often accompanied by a significant increase in intracellular calcium, contributing to inflammation progression [34]. Fluo-4 dye assays showed that LPS treatment significantly increased intracellular calcium, while CPP-CDs treatment suppressed this increase, restoring calcium balance (Fig. 5e). Using non-invasive microtest technique (NMT) to measure calcium influx, we confirmed that CPP-CDs significantly inhibited LPS-induced calcium influx (Fig. 5f, g and Supplementary Fig. 6c). This suggests that CPP-CDs maintain intracellular calcium homeostasis. Calcium influx is a key activator of NLRP3 inflammasome activation, further driving inflammation [35]. To determine whether CPP-CDs regulate NLRP3 activation by modulating calcium levels, we added calcium ion agonist Ionomycin to the CPP-CDs treatment group to elevate intracellular calcium. Ionomycin treatment reversed CPP-CDs’ inhibition of NLRP3 signaling and restored IL-1β secretion (Fig. 5h, i and Supplementary Fig. 6d), suggesting that CPP-CDs regulate NLRP3 activation by maintaining calcium homeostasis.
CPP-CDs modulate inflammatory signaling pathways. a. Western blot analysis of TLR4, p-P65, and P65 protein expression levels. BMDMs were treated with LPS (1 µg/mL, 4 h), low-concentration CPP-CDs (L, 0.5 mg/mL, 8 h), and high-concentration CPP-CDs (H, 1 mg/mL, 8 h). b. Immunofluorescence staining showing nuclear translocation of p-P65 in BMDMs (green) and DAPI-stained nuclei (blue) (n = 3 biological replicates). c. Western blot analysis of NLRP3, Cleaved-GSDMD, Pro-caspase-1, Cleaved-caspase-1, Pro-IL-1β, and Cleaved-IL-1β protein expression levels in BMDMs was performed using the same treatment as described in Fig. 5a. d. Immunofluorescence staining showing ASC speck formation and DAPI-stained nuclei (blue). BMDMs were treated with LPS (1 µg/mL, 4 h), Nig (5 µM, 45 min), and various concentrations of CPP-CDs (n = 3 biological replicates). e. Calcium ion influx in BMDMs measured using fluo-4 after LPS treatment for 10 min, with or without CPP-CDs pre-treatment for 6 h (n = 3 biological replicates). f, g. f (Upper panel): Net calcium flux measurements for different treatment groups using non-invasive microtest technology (NMT) in BMDMs, with each group tested for 5 min. g (Lower panel): Data are displayed as violin plots (n = 6 biological replicates). h. Western blot analysis of NLRP3, Cleaved-GSDMD, Pro-caspase-1, Cleaved-caspase-1, Pro-IL-1β, and Cleaved-IL-1β protein expression in BMDMs treated with LPS (1 µg/mL, 4 h), CPP-CDs (1 mg/mL, 8 h), and Ionomycin (5 µM, 1 h). i. ELISA measurements of IL-1β levels in BMDMs culture supernatants (n = 4 biological replicates). g, h, i. Data are expressed as mean ± standard error of the mean (SEM). One-way ANOVA was used for multiple group comparisons. *p < 0.05, **p < 0.01, ***p < 0.001
CPP-CDs regulate P2 X 7R/NLRP3 interaction and NLRP3 ubiquitination to modulate NLRP3 inflammasome activation
Next, we established an LPS/ATP-induced inflammation model to further investigate the additional potential anti-inflammatory mechanisms of CPP-CDs. Western blot analysis revealed that LPS and ATP treatment markedly upregulated the expression of P2 X 7R, a key ATP-binding receptor. However, CPP-CDs treatment significantly suppressed this upregulation, suggesting that CPP-CDs effectively attenuate P2 X 7R activation. (Fig. 6a and Supplementary Fig. 7a). Quantitative immunofluorescence analysis showed that after LPS/ATP treatment, YO-Pro-1 fluorescence intensity increased. However, CPP-CDs treatment significantly reduced YO-Pro-1 fluorescence intensity, suggesting that P2 X 7R activation was inhibited (Fig. 6b). P2X 7R is a key driver of NLRP3 inflammasome activation. Further analysis showed that CPP-CDs treatment significantly inhibited downstream NLRP3 signaling activation (Fig. 6c and Supplementary Fig. 7b), suggesting that CPP-CDs regulate downstream NLRP3 signaling by inhibiting P2X 7R activation. Additionally, we predicted the interaction between NLRP3 and P2 X 7R using the STRING database (Fig. 6d) and confirmed this relationship through immunoprecipitation experiments. Although NLRP3 expression did not change significantly, CPP-CDs treatment significantly reduced P2 X 7R expression, indicating that CPP-CDs reduce the P2 X 7R-NLRP3 interaction in a dose-dependent manner (Fig. 6e and Supplementary Fig. 7c, d). Furthermore, we used the protein synthesis inhibitor CHX to assess the degradation rate of NLRP3. The results showed that after 8 h of CHX treatment, the degradation rate of NLRP3 significantly increased (Supplementary Fig. 7e). While CPP-CDs treatment further accelerated NLRP3 degradation, rendering it more unstable during CHX treatment (Fig. 6f and Supplementary Fig. 7f). Immunoprecipitation experiments confirmed that CPP-CDs enhance NLRP3 ubiquitination. In the presence of the proteasome inhibitor MG132, NLRP3 ubiquitination levels were higher in the CPP-CDs treatment group (Fig. 6g and Supplementary Fig. 7g, h).
Regulation of P2X 7R/NLRP3 interaction by CPP-CDs. a. Western blot analysis of P2 X 7R protein expression in different treatment groups. BMDMs were treated with LPS (1 µg/mL, 4 h), ATP (5 mM, 30 min), low -concentration CPP-CDs (L: 0.5 mg/mL), and high-concentration CPP-CDs (H: 1 mg/mL) (n = 3 biological replicates). b. Left: YO-Pro-1 staining showing changes in BMDMs membrane permeability. Green represents YO-Pro-1 staining, and blue represents DAPI-stained nuclei. Scale bar: 50 μm. Right: Relative fluorescence intensity of YO-Pro-1 and DAPI (n = 3 biological replicates). c. Western blot analysis of NLRP3, Cleaved-GSDMD, Pro-caspase-1, Cleaved-caspase-1, Pro-IL-1β, and Cleaved-IL-1β protein expression levels in BMDMs treated as indicated in Fig. 6a (n = 3 biological replicates). d. Interaction network prediction of P2 X 7R and NLRP3 proteins using the STRING database, showing potential direct binding relationships. e. Immunoprecipitation assays detecting the interaction between P2 X 7R and NLRP3 in different treatment groups. BMDMs were treated with LPS (1 µg/mL, 6 h), ATP (5 mM, 30 min), and different concentrations of CPP-CDs (L and H) (n = 3 biological replicates). f. Western blot analysis of NLRP3 protein stability after treatment with Cycloheximide in BMDMs (Chx, 20 µg/mL, 8 h) (n = 3 biological replicates). g. Western blot analysis of NLRP3 ubiquitination levels after immunoprecipitation of NLRP3. Treatment conditions were LPS (1 µg/mL, 6 h), ATP (5 mM, 30 min), and different concentrations of CPP-CDs, with MG132 (10 µM, 6 h) used to block protein degradation pathways in BMDMs (n = 3 biological replicates)
The biocompatibility of CPP-CDs
CPP-CDs’ biocompatibility is critical for their potential applications. We administered 15 mg/kg and 30 mg/kg doses of CPP-CDs to mice via intraperitoneal injection every two days for 12 days, comparing with control groups (saline injection), and continuously monitored body weight changes (Fig. 7a). The results showed that neither treatment duration nor CPP-CDs dose affected mice body weight. At the end of the experiment, serum, BMDMs, and major organs (including colon, liver, spleen, lung, and kidney) were collected. In vitro, a CCK-8 assay evaluated the effect of CPP-CDs on cell proliferation over 72 h (Fig. 7b). The results showed no significant effects on BMDMs proliferation at these concentrations. Histological analysis of mouse organs showed no significant differences in tissue structure between the CPP-CDs treatment and control groups (Fig. 7c). Additionally, biochemical assays for liver function (ALT, AST) and kidney function (UA, UREA, CREA) showed normal levels (Fig. 7d-h). These results indicate that under the treatment conditions used in this study, CPP-CDs exhibit excellent biocompatibility, with no significant toxic effects, supporting their potential safety for biomedical applications.
Safety Assessment of CPP-CDs. a. Assessment of the impact of CPP-CDs administration on mouse body weight throughout the treatment period (n = 5, two independent experiments). b. Cytotoxicity evaluation of CPP-CDs on BMDMs using the CCK-8 assay. Cell viability was quantified at 0, 24, 48, and 72 h under different concentrations of CPP-CDs (0, 0.1, 0.5, and 1.0 mg/mL) based on OD450 values (n = 3, biological replicates). c. Morphological examination of the colon, liver, spleen, lung, and kidney tissues of mice from each group through H&E staining (magnification: 15×). d-h. Measurement of serum biomarkers, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), uric acid (UA), urea (UREA), and creatinine (CREA). This analysis assessed the effects of CPP-CDs on key physiological indicators (n = 4, biological replicates). a-b, d-h. Data are expressed as mean ± standard error of the mean (SEM). One-way or Two-way ANOVA was used for multiple group comparisons. *p < 0.05, **p < 0.01, ***p < 0.001