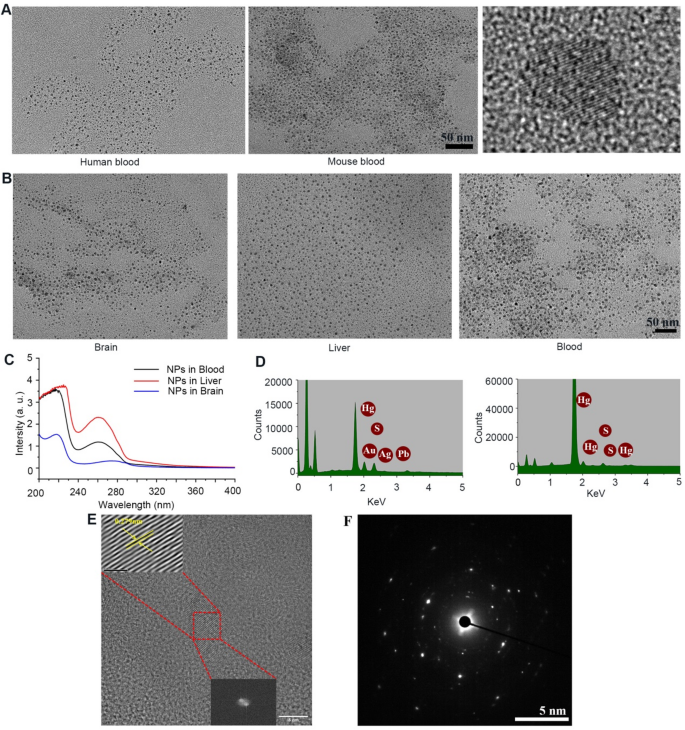
Structural characterization of HgS NPs synthesized in higher organisms
Tibetan medicine ZT is a blue-black powder made from mercury and sulfur through a tedious process similar to Bhasmas in Ayurvedic medicine. Notably, only sulfide forms of mercury are utilized in oral traditional medicines, and the chemical forms of mercury significantly influence their disposition, efficacy, and toxicity. Recent research has confirmed the good biocompatibility of ZnO NPs and Pt NPs auto-synthesized in animals and humans, demonstrating their potential in cancer-targeted therapy and imaging [35]. Given the propensity of metals or metal compounds to generate metal NPs in physiological environments owing to their physicochemical properties, we hypothesized that β-HgS in ZT might undergo instantaneous biosynthesis into HgS NPs in the blood, facilitating drug delivery and enhancing efficacy.
To test this hypothesis, blood samples from patients administered ZT (0.6 mg/kg) and mice administered ZT (3.0 mg/kg) or β-HgS (1.5 mg/kg) were collected 24 h post-oral ingestion and isolated by differential centrifugation ranging from 103 to 105 g at 4 °C. Encouragingly, transmission electron microscopy (TEM) revealed NPs with a diameter of 5–7 nm (Fig. 1A). Further confirmation of the presence of these NPs was obtained in the brain, liver, and kidney tissues of mice following cardiac perfusion, indicating their presence not only in the blood but also within parenchymal cells across various tissues (Fig. 1B). Notably, NPs were observed to traverse the BBB and enter the brain, potentially facilitating drug delivery into the brain. Importantly, these NPs were absent in ZT or β-HgS water solutions using the aforementioned isolation method, suggesting their biosynthesis by organisms rather than pre-existence in ZT or β-HgS (Fig. S1A, B). In addition, NPs in mice were purified further using HPLC (Fig. S1C-H), revealing UV spectroscopy absorption peaks at approximately 220 and 265 nm (Fig. 1C). The above results preliminarily confirmed that ZT/β-HgS were synthesized into uniform NPs by higher organism, but the elemental composition of nanoparticles still needed to be further clarified. Energy dispersive X-ray spectroscopy (EDS) confirmed the presence of Hg, Pb, Au, and S elements in NPs generated from ZT and Hg and S elements in nanoparticles generated from β-HgS (Fig. 1D), suggesting the possible synthesis of other metal salts in ZT into nanoparticles. High-resolution TEM analysis further confirmed the particle size distribution of captured nanoparticles ranging between 5 and 7 nm, with clear lattice fringes indicating a crystal plane spacing of 0.279 nm consistent with HgS nanoparticles. Selected area electron diffraction also revealed diffusion rings characteristic of nanoparticle crystals (Fig. 1E, F). These results confirmed that these nanoparticles should be HgS NPs.
As expected, HgS NPs could be rapidly formed and detected in various organs in mice within 30 min after the administration of ZT or β-HgS (Fig. S2). However, the synthesis speed of HgS NPs in vitro is significantly slower. When cells were treated with ZT (50 ng/mL) or β-HgS (25 ng/mL) for 6, 12, 24, 36, and 48 h, only minimal HgS NPs were detected at 24 h, with abundant and stable HgS NPs observed in both cells and culture medium after 36 h (Fig. S3). The lattice structure of HgS NPs biologically formed in vitro was more susceptible to disappearing under laser during TEM detection compared to that formed in vivo. We speculate that the protein coronas formed on the surface of HgS NPs in vivo and in vitro are different, playing a crucial role in the formation and stability of HgS NPs.
Purification and structural characterization of HgS nanoparticles (NPs) in vivo. (A) TEM images of unpurified NPs in the blood of patients and mice receiving oral administration of ZT/HgS (5–7 nm). (B) HgS nanoparticles in various tissues of mice after cardiac perfusion (Brain, Liver, Kidney). (C) UV absorption spectra of nanoparticles in different tissues purified by HPLC. (D) EDS spectrum of ZT and HgS NPs synthesized in mice. ZT NPs: Hg, S, Au, Ag, Pb; HgS NPs: Hg, S. (E) High-resolution TEM images of lattice fringes of HgS NPs (Interplanar spacing: 0.279 nm). (F) Selected area electron diffraction reveals diffusion rings in nanoparticle crystals
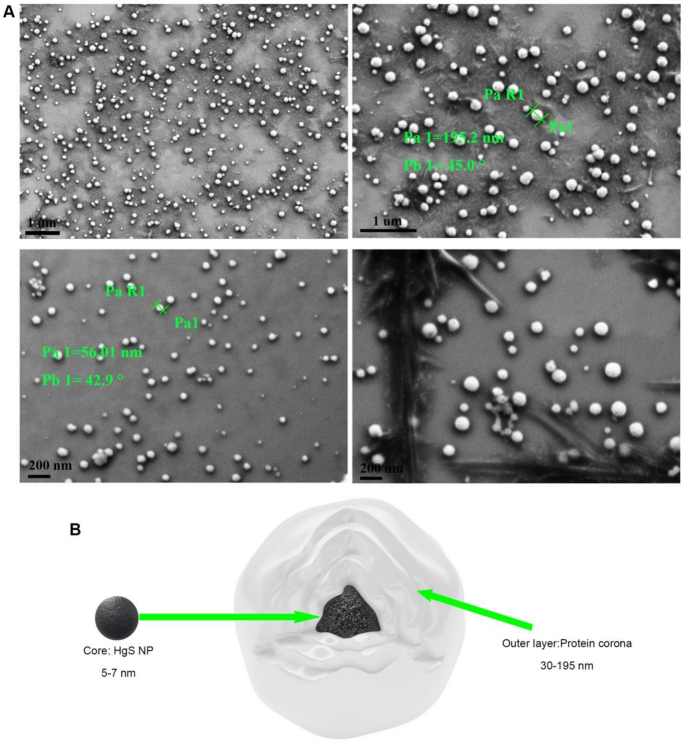
Morphology and composition of HgS NPs protein Coronas
In biological environments, the protein corona can be formed around the surface of nanomaterials instantaneously owing to the high surface free energy of nanomaterials [36, 37]. This biologically formed protein corona, in turn, can influence the biodistribution and therapeutic effects of the nanoparticles. Although the composition of the protein corona has been widely identified using HPLC-MS, its morphology remains a fundamental challenge, especially the morphology of autosynthesized nanoparticles in animals has not been reported so far [38, 39]. Recently, Sheibani and Kokkinopoulou have reported the morphology of the protein corona in vitro as an undefined and loose network of proteins using TEM [38, 39]. By contrast, we clearly detected the overall appearance of HgS NPs with protein coronas using field emission scanning electron microscopy (SEM) after HPLC purification from mice. The protein corona, as commonly supposed, forms a dense and layered shell coating the nanoparticle (Fig. 2, Fig. S4, S5). The HgS NPs with protein corona are spherical and roughly distributed in the range of 30–195 nm, considerably larger than the size of naked HgS NPs (Fig. 2A and B). Importantly, purification of large-sized nanoparticles from brain also challenges the conventional notion that nanoparticles within the size range of 5–100 nm are critical for crossing the BBB [40].
We further employed liquid chromatography mass spectrometry (LC-MS) to qualitatively and quantitatively identify the biologically relevant corona components. Over 200 different plasma corona proteins for HgS NPs were detected and quantified. Previous studies have mostly indicated that the protein corona is composed of only dozens of proteins, even in highly complex biological environments [12, 36, 41]. Tenzer recently reported that the protein coronas rapidly formed (< 0.5 min) on silica and polystyrene NPs in human plasma comprised nearly 300 different proteins [42]. Serum albumin is a major component of serum and has been found to be the main protein on the surface of some metal NPs such as Au NPs [43] and Pt NPs [12]. However, in this study, the main proteins on the surface of HgS NPs included hemoglobin subunit, transcriptional repressor, and superoxide dismutase, and albumin was rare (Table S1). Albumin is not the main protein of HgS NPs protein coronas, which may be related to the purification method and purity. The relatively thorough removal of miscellaneous proteins from tissues may be an important reason why we can clearly observe the complete structure of nanoparticles. The protein coronas formed on the surface of NPs might play essential roles in maintaining the drug delivery and targeting properties of the NPs.
ZT/β-HgS facilitate the penetration of drugs across BBB
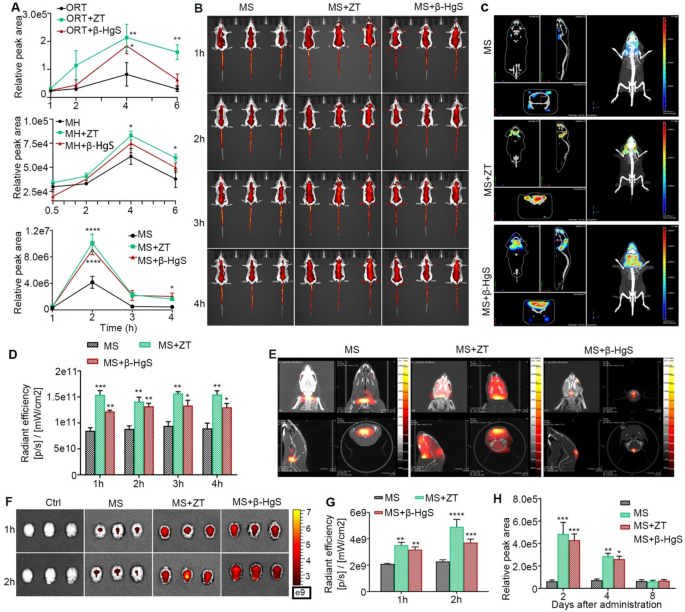
The increase of drug concentration in the brain is a compelling evidence for the capability of HgS NPs to facilitate drug penetration across the BBB. Hence, a series of qualitative and quantitative experiments were conducted to elucidate the role of HgS NPs in promoting drug entry into the brain in healthy mice (Fig. 3A). Initially, the contents of ORT, MH and MS-275 in the brain tissue of healthy mice at different time points were quantified using LC-MS. The contents of the three drugs in the brain of the combined administration groups were increased to varying degrees. After oral administration for 4 h and 6 h, the drugs contents in the brain of ORT combined administration groups and MH + ZT group were significantly higher than that of the single administration group. In particular, the combination of ZT/β-HgS with MS-275 resulted in significantly higher drug concentrations in brain tissue from 1 to 4 h compared to the administration of MS-275 alone, with the amount of MS-275 in the brain facilitated by ZT or β-HgS increasing by 1.5 times or 1.4 times, respectively, at 1 h, 2.4 times or 2.2 times, respectively, at 2 h, 4.4 times or 4.2 times, respectively, at 3 h, and 3.6 times or 4.5 times, respectively, at 4 h (Fig. 3A). These results confirm that ZT/β-HgS can promote the entry of drugs with different structures into the brain.
ORT and MH, as commonly used drugs in clinic, can readily pass through the BBB. However, as an inhibitor of HDAC3, a new target for Alzheimer’s disease, MS-275 has a brain entry rate of less than 10% [44]. Therefore, in order to better reflect the promoting effect of ZT/β-HgS on drug into the brain, MS-275 was selected as a control drug for further study [45,46,47]. The mice were orally administered with ZT (3 mg/kg)/β-HgS (1.5 mg/kg), followed by the intravenous injection of MS-275 at a dose of 20 mg/kg labeled with CY5.5 fluorophore after 4 h. Free MS-275 or MS-275 and ZT/β-HgS combination were administered to healthy mice, and the dynamic distribution of the drug in living tissue was imaged using an in vivo imaging system (IVIS) at intervals of 1, 2, 3, and 4 h. As anticipated, mice administered with MS-275 and ZT/β-HgS exhibited significantly higher fluorescence signals than those treated with MS-275 alone at 1–4 h throughout the entire body, including the head, suggesting that HgS NPs facilitate drug absorption in vivo, particularly across the BBB (Fig. 3B). Three-dimensional IVIS imaging and FLECT/CT preliminarily confirmed that although most of the fluorescence signals in the combined administration groups were still concentrated in the periphery of the brain, the signals entering into the brain had different degrees of enhancement compared with the administration of MS-275 alone. (Fig. 3C, D, E). To mitigate interference from blood or scalp in the brain, mice were anesthetized at 1 and 2 h post-administration, and brains were harvested after cardiac perfusion for IVIS imaging (Fig. 3F and G). Notably, combined administration of MS-275 and ZT/β-HgS exhibited significantly higher fluorescence signals in the brain compared to free MS-275 at 1 and 2 h, further confirming the ability of HgS NPs to facilitate drug transport across the BBB. These qualitative results further confirm that HgS NPs significantly promote drug accumulation in brain tissue. Similarly, using in vivo imaging, we also confirmed that ZT/β-HgS can promote drug delivery into the brain in zebrafish and rats (Fig. S6). In clinical Tibetan medicine treatment, ZT is typically administered once every 2–3 days. Therefore, its sustainability for synergistic drug action was evaluated. Mice were administered with ZT/β-HgS by gavage, followed by MS-275 administration after 2, 4, and 8 days, respectively, via intravenous injection. The drug concentration in the mice brain tissue was assessed 1 h post-dosing. As anticipated, the effect of promoting drug accumulation in the brain lasted for 4 days, with no significant effect observed on the 8th day, indicating that ZT/β-HgS exhibits a sustained effect in promoting drug transport across the BBB (Fig. 3H).
ZT/β-HgS promotes the drug to cross the BBB and enter the brain parenchyma. (A) Quantitative analysis of ORT, MH and MS-275 in the brain at different times after ZT/β-HgS combined with MS-275 administration by LC-MS (n = 5). (B) Bioluminescence images of whole mice treated with ZT or β-HgS combined with MS-275 at different time points show the accumulation of drugs in the brain (n = 3). (C) 3D reconstructed images displayed the distribution of MS-275 in the brain under different administration methods. In the combined administration groups, the fluorescence signals of the drug localized in the brain were significantly enhanced, not just in the muscles or skin. (D) Quantitative analysis of fluorescence signals of the drug in the whole-mouse brains. (E) CT images of the mouse brains at 2 h after ZT/β-HgS combined with MS-275 administration. (F, G) Bioluminescence images and quantitative analysis of brain tissues at 1 and 2 h after combined administration (n = 3). (H) The long-term effectiveness of ZT/β-HgS promotes the drug to cross the BBB. Mice were intravenously injected with MS-275 (20 mg/kg) on days 2, 4, and 8 after oral administration of ZT/β-HgS, and the concentrations of MS-275 in the brains after 2 h were measured by LC-MS. Data were presented as means ± SEM. compared with MS group, * p < 0.05, ** p < 0.01, *** p < 0.001
Synergistic effect of ZT/β-HgS on improving the learning and memory function in APP/PS1 mice
All the aforementioned findings demonstrate that ZT/β-HgS significantly facilitates drug entry into the brain across the BBB. These results prompted us to investigate the in vivo synergistic therapeutic effects on the CNS. The APP/PS1 double-transgenic mice model, characterized by memory and cognitive impairment, serves as an appropriate model for assessing the synergistic therapeutic effects of HgS NPs on the CNS. Although MS-275, a histone deacetylase (HDAC1 and HDAC3) inhibitor targeting a novel therapeutic pathway associated with memory and cognitive impairment, demonstrates some efficacy in improving the learning and memory abilities of APP/PS1 mice, its brain penetration rate is less than 10% [48]. In the experimental setup, model mice were administered with ZT (3.0 mg/kg)/β-HgS (1.5 mg/kg) via gavage and/or MS-275 (20 mg/kg) via intraperitoneal injection for a duration of two weeks. The learning and memory functions of the mice were assessed through three distinct behavioral experiments, including the open field test, Morris water maze (MWM), and Y-maze test.
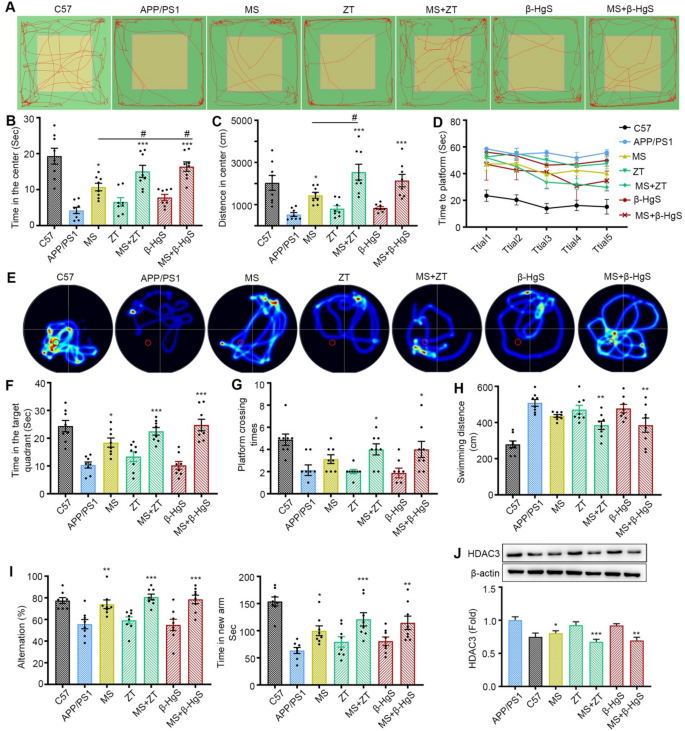
The open field test was employed to evaluate the exploratory behavior of mice across different administration groups on the 7th day of experimentation. In comparison to wild-type (WT) mice, the APP/PS1 mice exhibited markedly reduced spontaneous exploration behavior, as evidenced by shorter exploration times and paths in the central region (Fig. 4A). Conversely, mice treated with MS-275 alone or in combination with ZT/β-HgS displayed significant increases in exploration time and path length in the central region compared to model mice. Specifically, when compared to the group receiving MS-275 alone, mice administered with MS-275 and ZT demonstrated significantly greater exploration time or distance in the center, whereas no significant changes were observed in the ZT/β-HgS administration group. Although the combined administration of MS-275 and β-HgS also significantly increased the exploration time, there was no significant difference in the exploration distance in the center, which may be related to the running speed of the mice in this group (Fig. 4B, C). These findings suggest that ZT/β-HgS notably enhances the efficacy of the drug on the spontaneous exploration behavior of APP/PS1 mice.
The MWM test was conducted following 8 days of administration to assess the spatial learning and memory abilities of the mice. The spatial navigation task, involving the search for a hidden platform, was conducted from the 9th to the 13th day, followed by a probe trial on the 14th day after the removal of the hidden platform. Compared to WT mice, the APP/PS1 mice exhibited evident deficiencies in spatial learning and memory, as indicated by prolonged latency times to reach the platform, reduced activity, shorter time spent in the target quadrant post-platform removal, fewer platform crossings, and longer random swimming distances (Fig. 4D-H). Remarkably, ZT/β-HgS alone did not exert a noticeable effect on the spatial cognition and memory of APP/PS1 mice. However, following MS-275 treatment, the spatial cognition and memory abilities of the APP/PS1 mice were modestly improved. Specifically, mice treated with MS-275 in combination with ZT/β-HgS exhibited progressively shorter latency times over the course of training days (Fig. 4D), more focused search trajectories and longer durations spent in the target quadrant (Fig. 4E, F), increased platform crossings with an average of 4 times (Fig. 5G), and significantly shorter random swimming distances compared to APP/PS1 mice (Fig. 4H). These findings suggest that ZT/β-HgS significantly enhances the efficacy of the drug in improving the spatial learning and memory abilities of APP/PS1 mice.
Furthermore, Y-maze tests, comprising the free alternation experiment and exploration of the novel arm, were conducted to assess the mice’s propensity to explore new environments and their short-term working memory abilities. Mice treated with MS-275 in combination with ZT/β-HgS exhibited higher alternation rates and spent more time in the novel arm compared to the group treated with MS-275 alone, whereas no significant changes were observed in the ZT/β-HgS administration group (Fig. 4I).
Moreover, the expression levels of HDAC3, the target protein of MS-275, in the brain tissue of mice from each group were assessed via Western blot analysis. As depicted in Fig. 4J, the expression level of HDAC3 in the brains of APP/PS1 mice was significantly higher compared to that in WT mice. Notably, treatment with ZT/β-HgS alone did not induce any discernible change in HDAC3 expression. Conversely, mice administered with either the combined MS-275 and ZT/β-HgS or MS-275 alone exhibited a significant inhibitory effect on HDAC3 expression compared with APP/PS1. Remarkably, the combined administration demonstrated a stronger inhibitory effect on HDAC3 compared to administration of MS-275 alone, indicating that ZT/β-HgS could facilitate drug entry into the brain and enhance the inhibitory effect on the target protein. Overall, the results of several in vivo behavioral experiments collectively confirm that ZT/β-HgS can enhance the efficacy to some extent by promoting drug penetration across the BBB.
Synergistic effects of ZT/β-HgS on MS-275. (A-C) Open field test. The movement track of mice in the open field (A), the time (B) and distance (C) spent in the center of open field. (D-H), MWM tests spatial learning and memory abilities of mice. Escape latency (D), thermal images of mice swimming trajectories (E), time spent in the target quadrant (F), times of crossing the original location of the platform (G) and swimming distance of mice in the water tank (H). (I) Alternation rate and time spent in the new arm in Y-maze. (J) ZT/β-HgS combined treatment significantly reduced the expression of MS-275 target protein HDAC3 in mouse brain. n = 8 mice per group. Data were presented as means ± SEM. compared with APP/PS1 group, *p < 0.05, **p < 0.01, ***p < 0.001. Compared with MS group, # p < 0.05
ZT/β-HgS facilitate the uptake of drugs in HBMEC cells
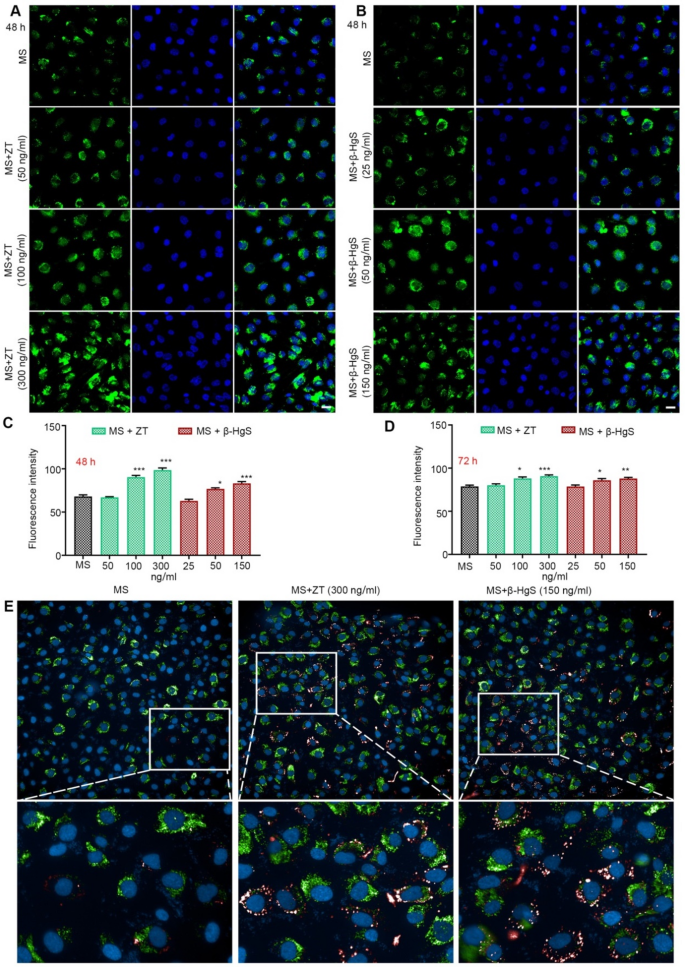
Brain microvascular endothelial cells (BMECs) constitute the main components of the BBB, responsible for transporting essential nutrients into the brain parenchyma and removing waste products from the brain. However, the majority of CNS drugs exhibit insignificant brain penetration (1-4%) due to low BBB permeability and/or rapid elimination [49]. Firstly, the cytotoxicity of MS-275, ZT, and β-HgS on human brain microvascular endothelial cells (HBMECs) was assessed, and the optimal dose for in vitro administration was determined (Fig. S7). Subsequently, we evaluated the effect of ZT/β-HgS on promoting drug entry into HBMECs. ZT (50, 100, and 300 ng/mL)/β-HgS (25, 50, and 150 ng/mL) was loaded with MS-275 (2 ng/mL) labeled with FITC fluorophore to image cellular uptake by confocal laser scanning microscopy (CLSM).
As expected, after incubating the cells with ZT/β-HgS and MS-275 for 48 h, the fluorescence intensity in cells treated with different doses of ZT/β-HgS was significantly enhanced compared with the group administered MS-275 alone, exhibiting a certain dose dependence (Fig. 5A-C, Fig. S8, S9). Results after 72 h of administration further confirmed that the intracellular fluorescence intensity of the combined administration group remained significantly higher than that of the single administration group (Fig. 5D and Fig. S10). Furthermore, drug accumulation at 48 h in the ZT/β-HgS high-dose groups was observed using a High-content screening system, with a substantial amount of drug accumulation and significant fluorescence signal enhancement clearly visible in the cytoplasm of live cells (Fig. 5E). These findings suggest that ZT/β-HgS could facilitate the transport of MS-275 into HBMECs.
Despite ZT/β-HgS has been confirmed to promote drug transport both in vitro and in vivo these findings, doubts persist regarding whether HgS NPs generated instantaneously in vivo can bind to drugs and facilitate drug delivery. To address this, after combined administration of ZT (3 mg/kg)/β-HgS (1.5 mg/kg) with MS-275 (20 mg/kg) in healthy mice for one day, the HgS NPs were separated from collected blood using differential centrifugation, varying from 103 to 105 g, at 4 °C, and the drug was extracted from the centrifugation product using dimethyl sulfoxide (DMSO) for subsequent detection by HPLC. However, no drugs were detected, indicating that the drug did not bind to the HgS NPs (Fig. S11). This suggests that the HgS NPs biologically formed in vivo may facilitate drug delivery in a stimulatory rather than carrier role.
ZT/β-HgS promotes the entry of MS-275 into HBMEC. (A–B) After 48 h of treatment with different drugs (MS (2 ng/mL), ZT (50, 100, 300 ng/mL) + MS (2 ng /mL), β-HgS (25, 50, 150 ng/mL) + MS (2 ng/mL)), the fluorescence intensity of the drugs in the HBMECs was observed by a confocal laser scanning microscope. MS-275 was labeled with FITC (green signal). Nuclei were stained with DAPI (blue signal). Scale bars, 50 μm. (C–D) Evaluation of intracellular drug fluorescence intensity in each treatment group at 48 and 72 h. (E) ZT (300 ng/mL)/β-HgS (150 ng/mL) combined with MS-275 incubation of HBMECs for 48 h, real-time observation of drug distribution and enrichment within live cells by High-content screening system (Operetta CLS, PerkinElmer, US). Data represent five independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001 versus MS
Proteomics suggests that ZT/β-HgS promotes vesicle transport
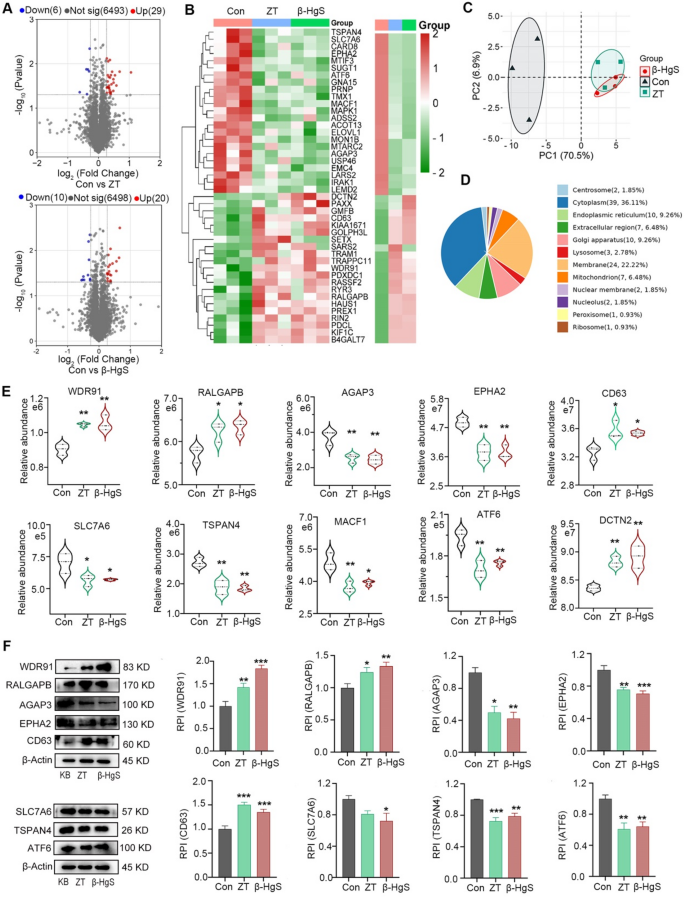
The above results confirm that ZT/β-HgS can be synthesized into nanoparticles and promote the drug into the brain to achieve synergistic effect, but deny the assumption that nanoparticles may be carriers. It is speculated that their mode of action should be to temporarily open the BBB or promote its active transport. To further clarify the possible mechanisms, we used proteomics analysis to investigate their regulatory effects on proteins in HBMECs. The results showed that compared with the control group, ZT significantly upregulated 29 proteins and downregulated 6 proteins, β-HgS significantly upregulated 20 proteins and downregulated 10 proteins, and 16 proteins were jointly regulated by both (Fig. 6A). From the analysis results of PCA and heatmap (Fig. 6B, C), it can be seen that β-HgS, as the main component of ZT, has a clear consistency with ZT in regulating proteins in cells. First of all, we noticed that there were no significant changes in intercellular tight junction and adhesion junction proteins, indicating that ZT/β-HgS may not promote drug delivery to the brain by temporarily interrupting BBB. Interestingly, most of these proteins are associated with intracellular and intercellular material transport. The results of subcellular organelle localization showed (Fig. 6D) that 24 proteins were localized on the cell membrane, and a large number of proteins were localized on the endoplasmic reticulum, lysosome and mitochondria. These organelles are involved in the synthesis and transport of intracellular substances. Among them, WDR91 and RALGAPB, which may be involved in regulating endocytosis and exocytosis [50,51,52,53,54,55,56,57], were significantly upregulated, while AGAP3, which inhibits the expression of caveolin [58], was significantly downregulated, and EPHA2, which promotes the expression of P-gp [59,60,61], was also significantly downregulated (Fig. 6E). The expression regulation of these proteins promotes the expression of key endocytosis proteins clathrin and caveolin, while reducing the expression of efflux protein P-gp, which may be related to ZT/β-HgS promoting drug entry into the brain. In addition, CD63, SLC7A6, and TSPAN4 were significantly downregulated, and they served as specific marker proteins for solute carrier, exosome, and migrasome [62,63,64,65,66,67,68], suggesting that ZT/β-HgS may also affect intracellular and intercellular vesicle transport. Meanwhile, the results also showed that MACF1, ATF6 and DCTN2 were also significantly regulated. These proteins are related to the structure and function of microtubules, which are the key supporting structures for vesicle transport [69,70,71]. We further verified the expression of these proteins (WDR91, RALGAP, AGAGP3, EPHA2, SLC7A6, TSPAN4, ATF6, DCTN2) by Western blotting, and confirmed that their expression changes in cells were basically consistent with the proteomic results (Fig. 6F). In general, ZT/β-HgS may not temporarily interrupt the tight junction of HBMECs, but have an important impact on the vesicular transport process of cells.
ZT/β-HgS affects the differential expression of proteome in HBMECs. (A) Volcano plots present the differentially expressed proteins of ZT and β-HgS compared with HBMECs in the control group (n = 3, FC > 1.2, P value < 0.05). Statistical analysis was performed with a two-tailed unpaired Student’s t test. Proteins with significantly increased and decreased expression are indicated by red and blue dots, respectively. (B) Heat map of cluster analysis of differentially expressed proteins in each group based on one-way ANOVA. Red and green represent proteins up-regulation and down-regulation, respectively. (C) Principal component analysis of all proteomes in different treatment groups. (D) Subcellular localization of 51 differentially expressed proteins based on GO functional annotation. The number and proportion of proteins on different subcellular organelles were annotated. (E) Effect of ZT and β-HgS on the expression of intracellular and intercellular transport related proteins. (F) Key differential proteins in proteomics were validated in vitro by Western blot and quantitative analysis. One way ANOVA was used to compare the differences between the treatment groups and the control group. n = 3, * P < 0.05, **P < 0.01
Mechanism of ZT/β-HgS on improving BBB permeability to drug
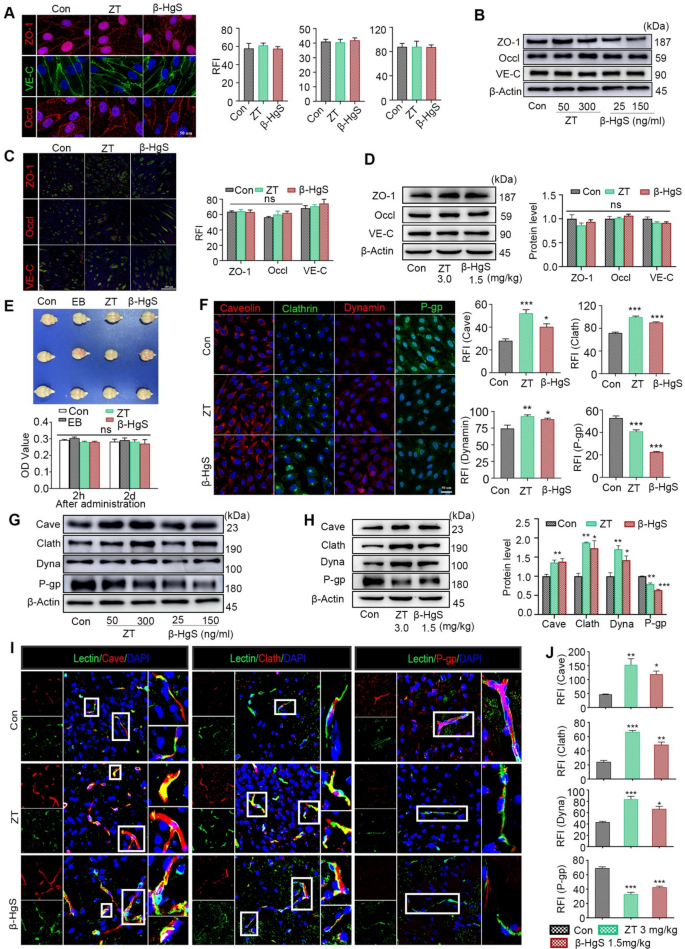
In order to further verify the results of proteomics, we studied the expression changes of BBB junction and endocytosis proteins in vivo and in vitro. The brain microvascular endothelial cells (BMECs) comprising the BBB are interconnected by intercellular junctions, notably tight junctions (TJs) and adherens junctions (AJs), which are crucial for maintaining BBB integrity and regulating its permeability. Degradation of TJs proteins such as occludin, AJs protein vascular endothelial (VE)-cadherin, and TJs accessory protein zonula occludens-1 (ZO-1) within BMECs can lead to capillary leakage, thereby increasing BBB permeability [72,73,74]. To explore whether HgS NPs facilitate the degradation of intercellular junctions within the BBB, we conducted immunohistochemistry and Western blot analyses for occludin, ZO-1, and VE-cadherin in vitro using human brain microvascular endothelial cells (HBMECs) treated with ZT/β-HgS for 48 h (Fig. 7A, B, Fig. S12, S13). Remarkably, no alterations in the distribution or expression levels of these proteins were observed in cells treated with ZT/β-HgS compared to normal cells. Consistent with the in vitro findings, in vivo experiments also demonstrated that the expression levels of occludin, ZO-1, and VE-cadherin in the brains of mice treated with ZT/β-HgS were comparable to those in normal mouse brains (Fig. 7C, D, Fig. S14). Both in vivo and in vitro results indicated that HgS NPs did not induce increased BBB permeability by damaging tight junctions and adherens junctions.
Additionally, the potential impact of HgS NPs on BBB disruption was evaluated through an Evans blue (EB) penetration assay. Mice were administered EB dye (2% w/v, 4 mL/kg) via tail vein injection at 4 h and 2 days after ZT/β-HgS administration (3.0 mg/kg, 1.5 mg/kg). The mice were euthanized 2 h after EB injection, and brain tissues were photographed and quantitatively analyzed following cardiac perfusion. As anticipated, no blue EB dye was detected in the brains of both normal mice and those administered with ZT/β-HgS (Fig. 7E). Quantitative analysis further confirmed that the absorbance values across all groups were similar, indicating that HgS NPs did not induce BBB disruption (Fig. 7E). These findings collectively suggest that HgS NPs facilitate drug accumulation in the brain without affecting the distribution and expression levels of intercellular junctions, thereby not leading to increased BBB permeability.
Then, whether ZT/β-HgS can enhance the permeability by regulating the transporters within the BBB? Caveolin-mediated or clathrin-mediated endocytosis are the primary pathways through which free drugs or nanoparticles can traverse the BBB and enter the central nervous system (CNS). Conversely, the efflux transporter P-glycoprotein (P-gp), expressed on both sides of the BBB, plays a crucial role in impeding potential therapeutics from crossing the BBB.
To examine whether HgS NPs induce increased BBB permeability by modulating transporters, we first assessed the impact of HgS NPs on key transcytosis proteins, including caveolin, clathrin, and dynamin, as well as the efflux protein P-glycoprotein (P-gp), in vitro using human brain microvascular endothelial cells (HBMECs). Following a 48-h incubation period with ZT/β-HgS, immunofluorescence imaging was conducted to evaluate the expression levels of caveolin, clathrin, dynamin, and P-gp in HBMECs using confocal laser scanning microscopy (CLSM). Compared to normal cells, those treated with ZT/β-HgS exhibited significantly enhanced fluorescence signals of caveolin, clathrin, and dynamin, indicating that HgS NPs could upregulate the expression of endocytosis proteins. Conversely, the fluorescence signals of the efflux protein P-gp in cells treated with ZT/β-HgS were notably reduced compared to normal cells, suggesting that HgS NPs could inhibit the expression of efflux proteins (Fig. 7F, Fig. S15). Moreover, the promotional and inhibitory effects of HgS NPs on endocytosis proteins and the efflux protein were confirmed by Western blot analysis (Fig. 7G, Fig. S16). Specifically, compared to normal cells, the expression levels of all three endocytosis proteins in cells treated with ZT/β-HgS were significantly upregulated, with approximately 68%/61%, 68%/34%, and 27%/12% increases in caveolin, clathrin, and dynamin, respectively, while the efflux protein level was markedly downregulated, with approximately 14%/22% decreases in P-gp.
The influence of HgS NPs on endocytosis proteins and the efflux protein of the BBB in healthy mice was also assessed. The mice received intragastric administration of ZT/β-HgS once every two days for three doses. Subsequently, the brains were collected for protein expression analysis after cardiac perfusion, and the cortex and hippocampus were isolated and processed for Western blotting analysis of protein levels. Western blotting analysis confirmed that the expression levels of caveolin, clathrin, and dynamin in the cerebral cortex and hippocampus were all significantly increased by approximately 34%/37%, 88%/73%, and 70%/42%, respectively, whereas the expression levels of P-gp were markedly reduced by 20%/36% (Fig. 7H). Moreover, immunohistochemical images revealed a significant increase in the distribution and fluorescence intensity of these three endocytosis proteins in brain microvessels, while the distribution and fluorescence intensity of the efflux protein P-gp in microvessels were notably decreased (Fig. 7I, J, Fig. S17). Furthermore, the caveolin and clathrin inhibitors genistein and chlorpromazine hydrochloride have been proven to eliminate the effect of ZT/β-HgS in promoting drug entry into HBMECs cells (Fig. S18). Overall, these findings are preliminarily confirmed that HgS NPs may facilitate drug penetration of the BBB into the brain by upregulating key endocytosis proteins and downregulating the efflux protein, rather than through temporary disruption of intercellular junctions in the BBB.
Effects of ZT/β-HgS on BBB permeability. (A) Effect of ZT/β-HgS on HBMECs intercellular connexins (ZO-1, occludin, and VE-cadherin) at 48 h. (B) Western blots did not show that the expressions of connexins in HBMECs were affected by ZT/β-HgS. (C) The expressions of connexins in brain were quantitatively analyzed by confocal laser scanning microscopy. (D) Western blots and quantitative analysis found that ZT/β-HgS had no effect on the expressions of connexins in mouse brain. (E) Physical disruption of the BBB was characterized by Evans blue (EB) penetration assay. (F) Effects of ZT/β-HgS on transporters in HBMECs were evaluated by immunocytochemistry (n = 3). (G–H) The expression of transporters in cells and brain were evaluated by Western blot (n = 3). (I–J) Effects of ZT/β-HgS on transporters in microvessels of BBB in mice (n = 6). Double-labeled immunostaining of caveolin, clathrin, dynamin and P-gp in mouse brain frozen sections. Target proteins were labeled with DyLight 594(Red). Nuclei were stained with DAPI (blue signal), and blood vessels were labeled with fluorescein isothiocyanate lectin (green signal). Data were presented as means ± SEM. Experiment performed at least three times. One-way ANOVA was used to analyze the differences between groups. * P < 0.05, **P < 0.01, ***P < 0.001 compared to Con
ZT/β-HgS facilitate the drug across BBB in the form of HgS NPs
The preceding results raise two distinct points. Firstly, ZT and β-HgS can be synthesized into uniform-sized HgS NPs both in vitro and in vivo. Secondly, ZT and β-HgS can facilitate drug penetration across the BBB to achieve synergy by modulating the expression of active transporters in the BBB. However, these processes are inherently separate, and the enhancement of drug transport across the BBB does not necessarily imply a direct correlation with HgS NPs. How then can we confirm whether this effect is indeed linked to HgS NPs or their synthesis process? One indirect approach involves examining whether the onset time of the observed effect aligns with the in vivo distribution of HgS and the synthesis time of HgS NPs.
Therefore, the quantification of Hg content in different tissues was assessed using atomic fluorescence spectrometer (AFS) analysis. The results revealed that the concentration of Hg in the brain continuously increased over time after oral administration of ZT/β-HgS, peaking at 20 ng/g at 24 h, and then gradually decreasing to 13.9 ng/g at 48 h, 5.65 ng/g at 96 h, and 4.41 ng/g at 192 h (Fig. S19A). The changing trend of Hg content in the brain mirrored the incremental trend of drugs in the brain, indicating a potential relationship between Hg or HgS NPs and the promotion of drug penetration across the BBB. On the 8th day, post-administration of ZT/β-HgS, the Hg content in the mouse brain rapidly decreased to near-normal levels, explaining the lack of drug increment after 8 days of ZT/β-HgS administration (Fig. S19B, C). Additionally, the changing trends of Hg content in the lung and blood mirrored those observed in the brain. However, in the liver and kidneys, the Hg concentration remained elevated on the 8th day, possibly due to metabolism (Fig. S19D, E). These results collectively demonstrate that promoting drug accumulation in the brain is positively correlated with the temporal distribution of Hg or HgS NPs in the brain.
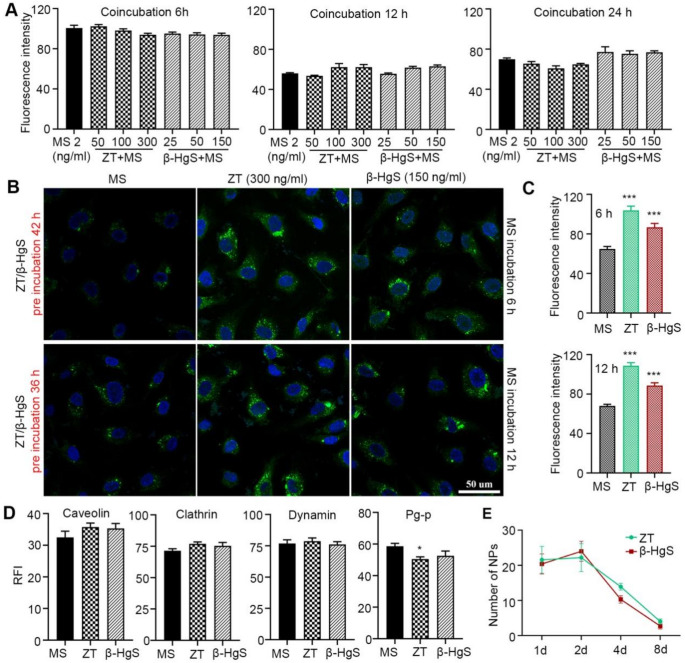
Further, the onset time of the increased cellular drug uptake capacity following combined administration was initially evaluated. Cells incubated with ZT/β-HgS and MS-275 exhibited nearly identical fluorescence signals at 6, 12, and 24 h compared to those treated with free MS-275 alone, indicating that ZT/β-HgS did not enhance the interaction between MS-275 and HBMEC cells (Fig. S20). Quantitative analysis revealed that the fluorescence intensity of the combined administration groups was slightly weaker than that of the single administration group, although not significantly different (Fig. 8A). These findings diverged markedly from the previous intracellular drug uptake results at 48 h, suggesting that ZT/β-HgS did not enhance drug entry into cells within the initial 24 h post-combined administration. Considering the biosynthesis speed of HgS NPs in cells discussed earlier, it is notable that the timeframe of ZT/β-HgS facilitating MS-275 entry into cells closely mirrored the time of HgS NPs formation. This observation indirectly suggests that ZT/β-HgS exerts a synergistic effect subsequent to the generation of HgS NPs. To further substantiate this inference, cells were pre-incubated with ZT/β-HgS alone for 36 and 42 h to ensure HgS NPs formation, followed by the addition of MS-275 labeled with FITC fluorophore to the pre-treated cells. As anticipated, cells pre-incubated with ZT/β-HgS exhibited significantly higher fluorescence signals at 6 and 12 h post-incubation with MS-275 compared to those without prior cultivation with ZT/β-HgS (Fig. 8B, C, Fig. S21).
In particular, the expression levels of transcytosis proteins and efflux protein also exhibited a close correlation with the generation time of HgS NPs. As depicted in Fig. 8D and Fig. S22, the expression levels of caveolin, clathrin, and dynamin proteins in cells treated with ZT/β-HgS showed no significant changes, and P-gp was not downregulated compared to normal cells at 24 h. However, significant changes in the expression levels of these proteins were observed at 48 h. These findings further indirectly support the notion that the changes in protein expression may be triggered by the generation of HgS NPs in the organism. Unlike the synthesis speed observed in vitro, the synthesis speed of HgS nanoparticles in mice is notably rapid, making it challenging to precisely track the onset time of their synergistic effects. However, their clearance speed is slow, and the presence or absence of nanoparticles in the brain may be correlated with their efficacy. The long-term efficacy of ZT and β-HgS has been previously confirmed, with the effect of facilitating drug transport across the BBB lasting for up to 4 days in vivo. If the effect is indeed related to HgS NPs, a substantial number of nanoparticles should be detected in the brain within the effective period. Conversely, when the effect diminishes, these nanoparticles should diminish or become insufficient to exert their function. To examine this hypothesis, brain tissues from mice were collected at 2, 4, and 8 days after administration of ZT or β-HgS, and the distribution of nanoparticles in the brain was assessed using TEM. The results revealed a substantial presence of nanoparticles in the brain tissue of mice on day 2 and day 4, whereas it was challenging to observe nanoparticles or only a few nanoparticles in the brain of mice on day 8 (Fig. 8E). The duration of existence of HgS NPs in the mouse brain closely aligns with the duration of the effect of ZT/β-HgS in promoting drug entry into the brain (as shown in Fig. 4H), further indirectly suggesting that the synergistic effect may indeed be related to HgS NPs or, at the very least, their synthesis process. These findings confirm that after ZT/β-HgS is auto-synthesized into HgS NPs by the organism, these nanoparticles promote enhanced transcytosis, thereby facilitating drug delivery across the BBB.
HgS NPs were the key to the synergistic effect of ZT/β-HgS. (A) ZT/β-HgS has no effect on drug entry into cells when no HgS NPs were synthesized within 6 to 24 h. (B) HBMEC were pre-incubated with ZT/β-HgS for 36 h and 42 h to ensure the formation of HgS NPs, and then after incubation with MS-275 for 6 h and 12 h respectively, the amount of drug entering the cells and the fluorescence intensity increased significantly. MS-275 was labeled with FITC (green signal). Nuclei were stained with DAPI (blue signal). Scale bar, 50 μm. (C–D) The expression levels of transcytosis proteins in cells incubated with ZT/β-HgS for 24 h. (E) Estimation of the number of HgS NPs in the brain. Data represent five independent experiments. * P < 0.05, ***P < 0.001 compared to Ms