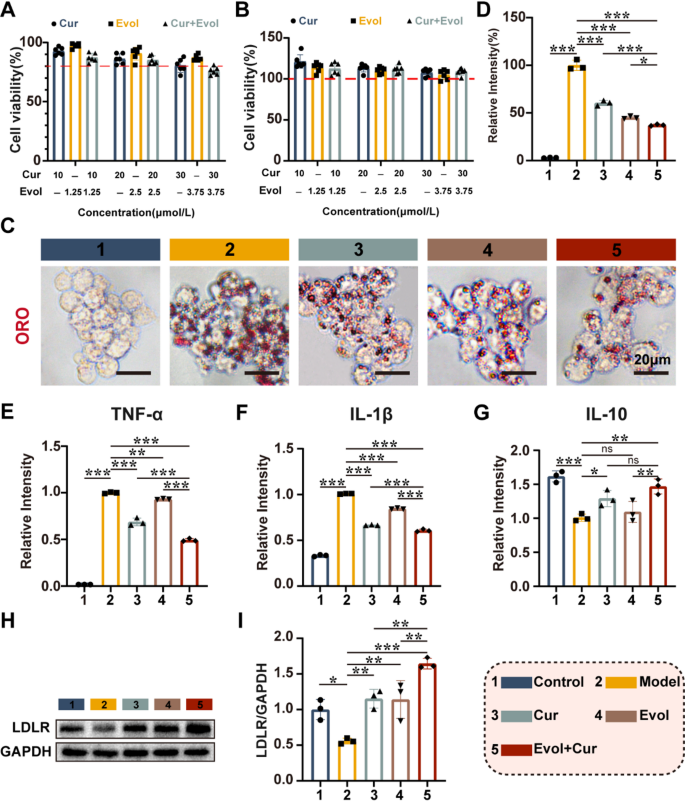
The synergistic effects of Evol and Cur in anti-inflammatory and lipid regulation
Previous studies have reported that high Hcy levels can impair lipid efflux from macrophages and enhance foam cell formation, accompanied by the development of an inflammatory response, which cooperately contribute to the atherosclerosis development [23, 24]. More dangerously, if the liver cannot clear the excreted lipids on time, the aggravate accumulation of cholesterol in the blood will further promote the progression of atherosclerosis [24]. Evol can promote macrophage lipid efflux and reduce foam cell formation. Furthermore, it increases the recycling of LDLR, which is important for plasma LDL-C clearance [12, 25, 26]. Cur has been shown to inhibit the inflammatory response [15]. Thus, we investigated the potential of Evol and Cur as therapeutic agents for Hcy induced AS therapy. MTT assay showed that cell viability varied little with increasing concentrations of the administered drugs. Therefore, low concentrations of Curcumin (10 µM) and Evolocumab (1.25 nM) were used to mitigate drug toxicity in the subsequent exploration (Fig. 1A&B). Under these conditions, the co-administration significantly enhanced cholesterol efflux from macrophages, which demonstrated the superior ability of the combination in reducing macrophage lipid accumulation (Fig. 1C&D). ELISA assay indicated that co-administration of Evol and Cur showed the inhibitory effect on typical inflammatory cytokines of TNF-α and IL-1β, while simultaneously promoted the expression of anti-inflammatory factors IL-10. (Fig. 1E-G). Western blot demonstrated that the co-administration significantly upregulated the levels of LDLR, compared to the sole administration of Evol, which is important for the subsequent plasma cholesterol scavenging by LDLR cholesterol (Fig. 1H&I). Hence, we conclude that the combination of Evol and Cur can inhibition macrophage lipid accumulation and reduce inflammation, while increasing hepatocyte LDLR expression.
The synergistic effects of Evol and Cur in anti-inflammatory and lipid regulation. (A&B) Effect of different concentrations of Curcumin and Evolocumab on cell viability of activated macrophages and hepatocytes; (C&D) Light microscopy images of activated macrophages internalizing ox LDL. [Hcy] = 100 µM, [OxLDL] = 80 µg/mL, [Cur] = 10 µM, [Evol] = 1.25 nM, RAW264.7 cells were sequentially incubated with Hcy for 24 h and ox LDL for 48 h, Scale bar = 20 μm; (E–G) ELISA analysis of TNF-α, IL-1β and IL-10; (H&I) Western blot analysis of LDLR. Data are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.001, ***P < 0.0001. ns, not significant
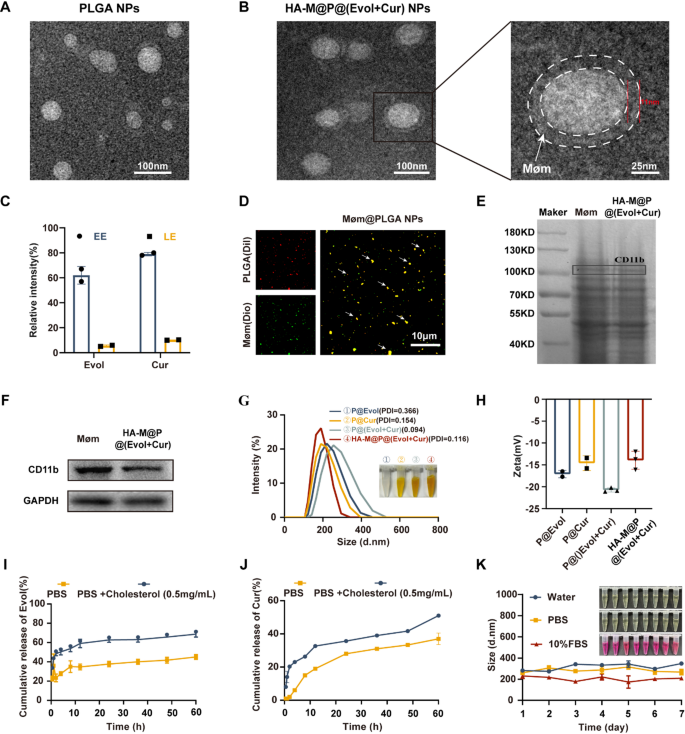
Preparation and characterization of HA-M@P@(Evol + Cur) NPs
Transmission Electron Microscopy (TEM) images revealed that the PLGA nanoparticles exhibit a spherical morphology (Fig. 2A). In contrast, the HA-M@P@(Evol + Cur) NPs demonstrated a unique core-shell architecture characterized by P@(Evol + Cur) NPs as the inner core surrounded by an approximately 11-nm-thick outer membrane layer (denoted by white dashed circles). (Fig. 2B). Leveraging the hydrophobic core, Evol and Cur were successfully encapsulated at drug-to-PLGA NPs ratios of 1:20 and 1:10, achieving encapsulation efficiencies (EE%) of 60.0% and 80.0%, with drug loading efficiencies (LE%) reaching 5% and 10%, respectively (Fig. 2C). Fluorescence imaging was employed to validate the nanoparticle coating. Briefly, PLGA NPs were loaded with the fluorescent dye 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate (DiI, red), which were then fused with 3,3’-dioctadecyloxacarbocyanine perchlorates (DiO, green)-labeled Møm to obtain Møm@PLGA NPs. As shown in Fig. 2D, the merged CLSM image exhibited bright yellow fluorescence signals, representing the co-localization of green and red fluorescence, which conclusively demonstrated successful coating of PLGA NPs with Møm. Subsequently, colocalization analysis between Møm (green) and PLGA NPs (red) was performed using ImageJ software via Pearson’s coefficient and Manders’ coefficient. The coefficient ranges from 0 to 1, representing no overlap and complete overlap, respectively [27]. The higher values indicate more nanoparticles being enveloped by the membrane. As shown in Fig. S1, Pearson’s correlation coefficient is 0.872, and the Manders’ correlation coefficient is 0.736, indicating that the nanoparticles are effectively wrapped by the macrophage membrane. SDS-PAGE analysis confirmed the integrity of membrane proteins in both Møm and HA-M@P@(Evol + Cur) NPs (Fig. 2E). Western blot analysis further verified the presence of the Møm-specific marker CD11b in HA-M@P@(Evol + Cur) NPs (Fig. 2F). Dynamic light scattering (DLS) measurements indicated that HA-M@P@(Evol + Cur) NPs had an average diameter of 216.5 nm (Fig. 2G) and a zeta potential of -13.9 mV (Fig. 2H). These results showed successful preparation of HA-M@P@(Evol + Cur) NPs. To simulate atherosclerotic lesion conditions, drug release experiments were conducted in PBS containing 0.5 mg cholesterol [28]. In the absence of cholesterol, the cumulative release rates of Evol and Cur reached 45% and 37% respectively within 60 h, determined by combining with standard calibration curves (Fig. S2A&B). Remarkably, in cholesterol-enriched conditions, the release rates increased significantly to 68.6% for Evol and 51% for Cur at the same time point (Fig. 2I&J). These findings indicate that the hypercholesterolemic environment substantially enhances drug release from HA-M@P@(Evol + Cur) NPs, thereby improving the efficacy against atherosclerosis. Meanwhile, after incubation in water, PBS, and culture medium containing 10% fetal bovine serum (FBS) for up to 7 days, the particle size distribution of HA-M@P@(Evol + Cur) NPs was found to remain stable (Fig. 2K), underscoring their potential for in vivo applications.
Characterization of HA-M@P@(Evol + Cur) NPs. (A) TEM image of PLGA NPs; (B) TEM photographs and enlargements of HA-M@P@(Evol + Cur) NPs; (C) Evol and Cur entrapment efficiency and loading capacity of HA-M@P@(Evol + Cur) NPs; (D) Confocal fluorescent microscopy images of Møm@PLGA NPs (red = PLGA, green = Møm, scale bar = 10 μm); (E) SDS-PAGE analysis of protein bands obtained for Møm and HA-M@P@(Evol + Cur) NPs; (F) SDS-PAGE analysis of retention protein bands of Møm and HA-M@P@(Evol + Cur) NPs; (G&H) Particle size and zeta potential of HA-M@P@(Evol + Cur) NPs analyzed by DLS; (I&J) Cumulative release of Evol(I) and Cur(J) from nanocomplexes in PBS with/without cholesterol; (K) The stability of HA-M@P@(Evol + Cur) NPs when incubated for 7 days in water, PBS and medium containing 10% fetal bovine serum
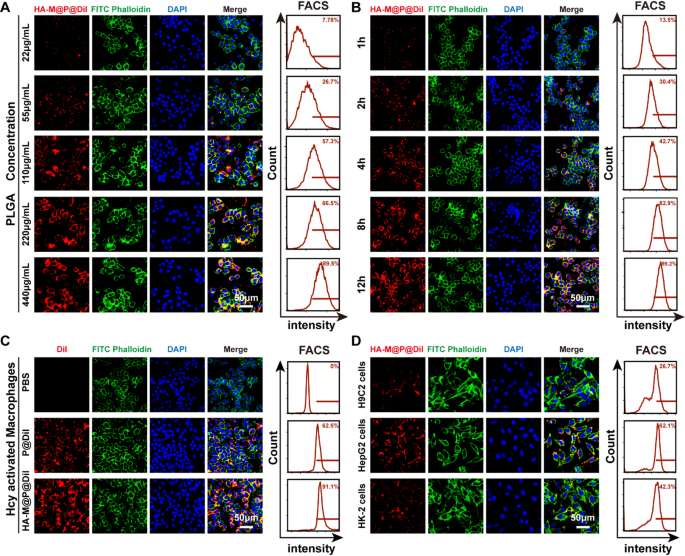
Cellular uptake and targeting ability of HA-M@P@(Evol + Cur) NPs in vitro
In this study, we systematically evaluated the cellular uptake and targeting ability of HA-M@P@(Evol + Cur) NPs. To track the distribution of nanoparticles, the fluorescent dye Dil was employed as a tracer. PLGA nanoparticles labeled with Dil were camouflaged with Møm and modified with HA as a targeting molecule, resulting in the preparation of HA-M@P@Dil NPs. An in vitro atherosclerosis model was successfully established by treating Raw 264.7 cells with 100 µM Hcy. The uptake behavior of HA-M@P@Dil NPs by activated macrophages (Hcy-stimulated Raw 264.7 cells) was qualitatively and quantitatively analyzed using immunofluorescence staining and flow cytometry. The results demonstrated that the cellular uptake efficiency of HA-M@P@Dil NPs exhibited dose- and time-dependent characteristics, with the red fluorescence intensity of Dil significantly increasing as the PLGA concentration and incubation time increased. Flow cytometry analysis further revealed that when the PLGA concentration reached 220 µg/mL, the cellular uptake rate was 66.5%; extending the incubation time to 12 h resulted in an uptake rate of 95.2%, approaching saturation (Fig. 3A&B). In Fig. 3C, compared to the P@Dil NPs group, distinct Dil-labeled red fluorescence was observed in the HA-M@P@Dil NPs group. Further flow cytometry analysis indicated that activated macrophages exhibited markedly higher uptake efficiency for HA-M@P@Dil NPs (91.1%), compared to P@Dil NPs (62.5%). This result demonstrated the superior homing capability of HA-M@P@Dil NPs toward activated macrophages due to the interaction between hyaluronic acid (HA) and CD44 on activated macrophage surfaces [29]. To verify the HA-dependent targeting mechanism, we performed competitive inhibition experiments through HA pretreatment (Fig. S3A&B). The results showed that pre-incubation with HA solution prior to HA-M@P@Dil NPs administration significantly reduced fluorescence signal intensity in macrophages (approximately 52% reduction), confirming the critical role of HA in macrophage targeting.
To elucidate the internalization mechanism of the nanodelivery system, three specific endocytosis inhibitors were employed: colchicine (inhibiting micropinocytosis), chloroquine (inhibiting clathrin-mediated endocytosis), and methyl-β-cyclodextrin (M-β-CD, inhibiting caveolae-mediated endocytosis). Pretreatment with inhibitors showed that in the presence of 8 µg/mL colchicine and 120 µg/mL chloroquine, the cellular fluorescence signal was significantly attenuated (Fig. S4A&B), indicating that HA-M@P@(Evol + Cur) NPs primarily enter cells through micropinocytosis and clathrin-mediated endocytosis pathways, a mechanism that may be closely related to their anti-atherosclerotic effects. Furthermore, the study systematically evaluated the cellular uptake characteristics of the nanoparticles across different cell lines. The results showed that HA-M@P@Dil NPs exhibited the highest uptake efficiency in HepG2 cells (human hepatocarcinoma cell, 62.1%), significantly higher than that of H9C2 cells (rat cardiomyocytes cell, 26.7%) and HK-2 cells (human renal tubular epithelial cell, 42.3%) (Fig. 3D). In contrast, P@Dil NPs demonstrated significantly lower cellular uptake rates across all three cell types (Fig. S5). These findings suggest that activated macrophages and hepatocytes have a higher uptake capacity for HA-M@P@Dil NPs, providing experimental evidence for the anti-atherosclerotic effects of HA-M@P@(Evol + Cur) NPs in different tissue sites in vivo.
Cellular uptake of HA-M@P@(Evol + Cur) NPs. (A) Confocal images and flow cytometry analysis of activated macrophages incubated with the different doses of HA-M@P@Dil NPs for 12 h; (B) Confocal images and flow cytometry analysis of activated macrophages incubated with the different doses of HA-M@P@Dil NPs (220 µg/mL PLGA) for 1, 2, 4, 8 and 12 h; (C) Confocal images and flow cytometry analysis of activated macrophages incubated with P@Dil NPs and HA-M@P@Dil NPs (220 µg/mL PLGA) for 12 h; (D) Confocal images and flow cytometry analysis of H9C2, HepG2 and HK-2 cells after incubation with HA-M@P@Dil NPs for 12 h, DAPI, phalloidin and DiI were used to indicate nuclei, actin filament, and different NPs, respectively. All scale bars = 50 μm
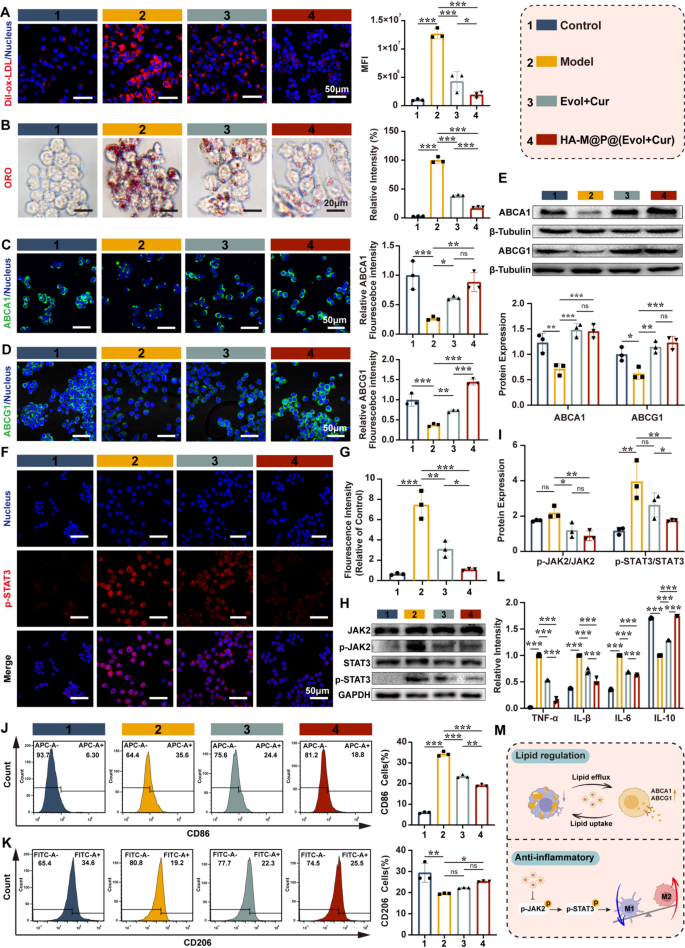
HA-M@P@(Evol + Cur) NPs regulate lipid homeostasis and attenuate inflammation of macrophages
Previous studies have reported that homocysteine (Hcy) can increase lipid uptake in macrophages and promote the occurrence of inflammation [30]. Therefore, we used Dil-oxLDL as a tracer to evaluate the effect of HA-M@P@(Evol + Cur) NPs on lipid deposition. The results showed that activated macrophages exhibited significantly enhanced lipid uptake, as indicated by stronger red fluorescence signals from Dil-oxLDL in the activated macrophage group compared to the Evol + Cur and HA-M@P@(Evol + Cur) NPs groups (Fig. 4A). Analogously, Oil Red O (ORO) staining further confirmed that, compared to the model group, both the Evol + Cur and HA-M@P@(Evol + Cur) NPs groups effectively inhibited ox-LDL uptake, reducing lipid droplet formation in activated macrophages by 2.6-fold and 5.9-fold, respectively (Fig. 4B). What’s more, Hcy leads to a reduction in lipid efflux in macrophages through an ABCA1/ABCG1-dependent mechanism, thereby accelerating the formation of foam cells and promoting the pathological progression of atherosclerosis [5, 12]. Based on this, our study investigated the effect of Hcy on the expression of ABCA1 and ABCG1. Immunofluorescence staining results revealed that, compared to the control group, Hcy significantly suppressed the green fluorescence intensity of ABCA1 and ABCG1 in macrophages (Fig. 4C&D), a finding consistent with our previous research [31]. Further Western blot analysis revealed that treatment with HA-M@P@(Evol + Cur) NPs significantly upregulated the protein expression levels of ABCA1 and ABCG1 (Fig. 4E), demonstrating their ability to effectively reverse Hcy-induced impairment of cholesterol efflux in macrophages. These findings suggest that HA-M@P@(Evol + Cur) NPs can inhibit lipid accumulation in macrophages by reducing lipid uptake and enhancing lipid efflux.
In addition, Hcy was reported to activate the inflammatory response of macrophages and increase secretion of inflammatory factors through JAK2/STAT3 signaling pathway [23]. The elevated level of inflammatory factors will further interfere with lipid metabolism in macrophages, aggravating the development of atherosclerosis [32]. To investigate the anti-inflammatory effects of HA-M@P@(Evol + Cur) NPs, we first established an inflammatory macrophage model using Hcy-stimulated macrophages. Detection with the fluorescent probe 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) revealed prominent green fluorescence signals in the model group, whereas the fluorescence signals were significantly attenuated in the Evol + Cur and HA-M@P@(Evol + Cur) NPs treatment groups (Fig. S6A). Flow cytometry results further confirmed that HA-M@P@(Evol + Cur) NPs effectively reversed the abnormal elevation of reactive oxygen species (ROS) in inflammatory macrophages (Fig. S6B).
As a key regulatory factor in inflammatory signaling pathways, signal transducer and activator of transcription 3 (STAT3) plays a crucial role in the progression of atherosclerosis [33]. Its phosphorylated form (p-STAT3) translocate to the nucleus, promoting the transcription of inflammation-related genes. Immunofluorescence experiments revealed that, compared to the model group, the HA-M@P@(Evol + Cur) NPs group exhibited significantly reduced levels of p-STAT3 (Fig. 4F&G). Western blot analysis further confirmed that, compared to the model group, the HA-M@P@(Evol + Cur) NPs group markedly downregulated the expression of p-STAT3 and its upstream protein p-JAK2 without affecting the total protein levels of JAK2 and STAT3 (Fig. 4H&I). Given the involvement of the JAK2/STAT3 signaling pathway in regulating macrophage polarization [34], we assessed the expression of the M1 macrophage marker iNOS (red) and the M2 macrophage marker Arg-1 (green) using immunofluorescence. The results showed that the model group displayed high-intensity red fluorescence and low-intensity green fluorescence, whereas HA-M@P@(Evol + Cur) NPs treatment significantly reversed this pattern (Fig. S7A&B). Notably, Western blot analysis demonstrated that, compared to the model group, the Evol + Cur and HA-M@P@(Evol + Cur) NPs groups exhibited lower iNOS levels and higher Arg-1 expression (Fig. S8A&B). Flow cytometry analysis revealed that the proportion of CD86 + macrophages in the HA-M@P@(Evol + Cur) NPs group (18.8%) was significantly lower than that in the Evol + Cur group (24.4%) and the Hcy-induced group (35.6%), while the proportion of CD206 + macrophages (25.50%) was higher than that in the Evol + Cur group (22.3%) and the model group (19.2%) (Fig. 4J&K). To further validate the expression of inflammatory cytokines, ELISA assays were performed to measure the levels of TNF-α, IL-1β, IL-6, and IL-10. The results showed that HA-M@P@(Evol + Cur) NPs significantly reduced the levels of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) while increasing the expression of the anti-inflammatory cytokine IL-10 (Fig. 4L). In summary, HA-M@P@(Evol + Cur) NPs can effectively delay the pathological progression of atherosclerosis by regulating lipid homeostasis and modulating the inflammatory response (Fig. 4M).
HA-M@P@(Evol + Cur) NPs regulate lipid homeostasis and attenuate inflammation of macrophages. (A) Confocal fluorescence images and semi-quantitative analysis of Dil-oxLDL internalization in RAW264.7 cells, Scale bar = 50 μm; (B) Optical microscopy images and semi-quantitative analysis of ORO staining, Scale bar = 20 μm; (C&D) Confocal fluorescence images and quantitative analysis of ABCA1 and ABCG1 in activated macrophages under different treatments, Scale bar = 50 μm; (E) Western blot analysis of ABCA1 and ABCG1 in activated macrophages subjected to different treatments; (F&G) Confocal fluorescence images and quantitative analysis of p-STAT3 in activated macrophages under different treatments, Scale bar = 50 μm; (H&I) Western blot analysis of p-JAK2, JAK2, p-STAT3 and STAT3 on activated macrophages with different treatment; (J&K) Flow cytometry profiles of M1 phenotype highly expressed CD86 and M2 phenotype highly expressed CD206; (L) ELISA assay of TNF-α, IL-1β,IL-6 and IL-10; (M) Schematic Diagram of Lipid Homeostasis Regulation and Anti-inflammatory Mechanisms of HA-M@P@(Evol + Cur) NPs. Data are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.001, ***P < 0.0001. ns, not significant
HA-M@P@(Evol + Cur) NPs promote LDLR expression in hepatocyte and thereby enhance plasma cholesterol clearance
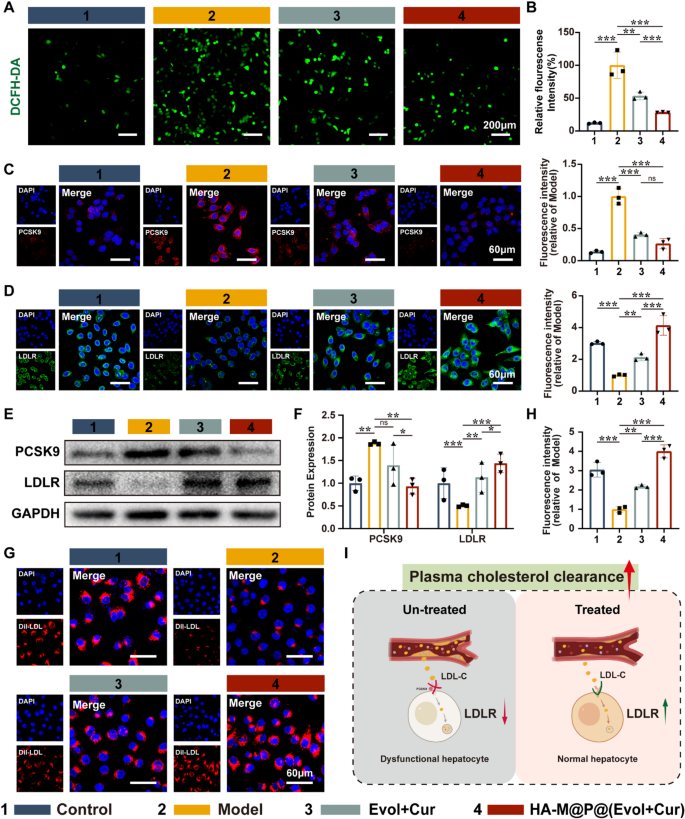
Dyslipidemia is a significant factor that influences the progression of AS. Previous studies have shown that Hcy induces hepatic oxidative stress, resulting in disrupted intrahepatic lipid metabolism and elevated blood LDL-C levels, thereby accelerating AS progression [35,36,37]. To observe the generation of reactive oxygen species (ROS) in hepatocytes induced by Hcy, we employed the fluorescent probe 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) for detection. The results demonstrated a significant increase in ROS levels in the model group compared to the control group, as evidenced by the intense green fluorescence emitted by the DCFH-DA probe. In contrast, both Evol + Cur and HA-M@P@(Evol + Cur) NPs reversed the high fluorescence signal from DCFH-DA, with HA-M@P@(Evol + Cur) NPs demonstrating a more pronounced advantage (Fig. 5A&B). This suggests that HA-M@P@(Evol + Cur) NPs effectively ameliorate Hcy-induced oxidative stress in hepatocytes.
Published studies have confirmed that PCSK9 is a key regulator of lipid homeostasis, binding to the LDL receptor (LDLR) on the surface of hepatocytes and preventing its recycling and promoting its degradation in lysosomes [38]. LDLR primarily mediates the endocytosis of cholesterol-rich LDL, and its dysfunction leads to elevated plasma cholesterol levels, thereby exacerbating AS progression [39]. Based on this, we utilized fluorescence imaging to assess the expression levels of PCSK9 and LDLR in cells. As expected, the red fluorescence intensity of PCSK9 was significantly increased in the model group compared to the control group, while the green fluorescence intensity of LDLR was markedly reduced. Notably, after intervention with HA-M@P@(Evol + Cur) NPs, the expression level of PCSK9 decreased by approximately 74% compared to the model group, while the expression of LDLR on the hepatocyte surface increased by about 76% (Fig. 5C&D). These findings were further confirmed by Western blot analysis (Fig. 5E&F).
To further investigate the function of LDLR, we co-incubated hepatocytes with Dil-labeled LDL. The results showed that the red fluorescence of Dil was significantly attenuated in the model group, whereas the uptake of LDL was markedly enhanced in hepatocytes treated with Evol + Cur and HA-M@P@(Evol + Cur) NPs, with the HA-M@P@(Evol + Cur) NPs group showing an approximately 31.3% improvement compared to the Evol + Cur group (Fig. 5G&H). These data indicate that HA-M@P@(Evol + Cur) NPs effectively promote the recycling and reuse of LDLR, thereby enhancing the clearance efficiency of serum cholesterol and ultimately exerting anti-atherosclerotic effects [40].
HA-M@P@(Evol + Cur) NPs promote LDLR expression in hepatocyte and thereby enhance plasma cholesterol clearance. (A&B) Representative confocal images and quantitative analysis of ROS generation in hepatocytes detected by redox-sensitive probe DCFH-DA, scale bar = 200 μm; (C&D) Confocal images and quantitative analysis of PCSK9 and LDLR in hepatocytes after different treatments, scale bar = 60 μm; (E&F) Protein blotting analysis of PCSK9 and LDLR expression in hepatocytes after different treatments and quantitative analysis; (G&H) Confocal images and quantitative analysis of Dil-LDL internalization by hepatocytes after different treatments, scale bar = 60 μm; (I) Schematic illustration of HA-M@P@(Evol + Cur) NPs enhancing the uptake of circulating LDL by hepatocytes. Data are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.001, ***P < 0.0001. ns, not significant
Pharmacokinetics and targeting of HA-M@P@(Evol + Cur) NPs
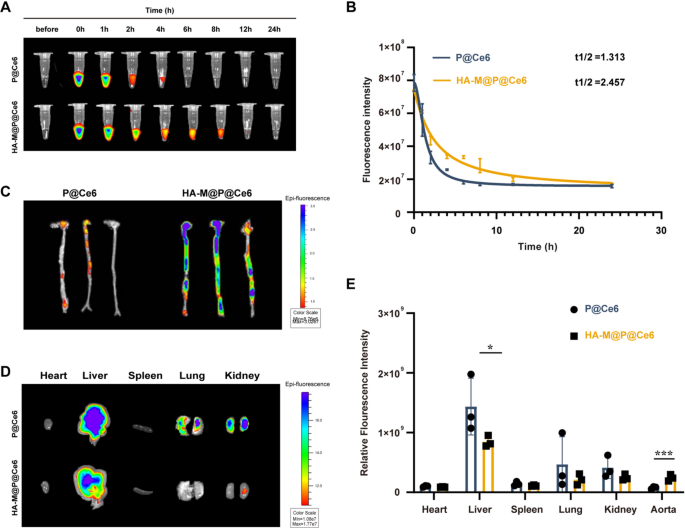
To investigate whether HA-M@P@(Evol + Cur) NPs can effectively prolong their circulation time in wild-type C57BL/6 mice, we employed pharmacokinetic analysis. The core of this method involves using labeling techniques to track the in vivo dynamics of nanoparticles. Experimental data revealed that the half-life of P@Ce6 was 1.313 h, whereas the half-life of HA-M@P@(Evol + Cur) NPs was significantly extended to 2.457 h (Fig. 6A&B). This finding confirms that the biomimetic membrane coating effectively reduces immune clearance, thereby prolonging the circulation time of nanoparticles [41]. Undoubtedly, the extended circulation time facilitates the accumulation of drugs in target tissues.
To further assess the targeting performance of nanoparticles, experiments were conducted using ApoE−/− atherosclerosis model mice, with intravenous administration of P@Ce6 NPs and HA-M@P@Ce6 NPs, respectively. Fluorescence imaging revealed that HA-M@P@Ce6 NPs exhibited approximately threefold higher signal intensity in the aortic region compared to P@Ce6 NPs (Fig. 6C), indicating that hyaluronic acid (HA) modification combined with biomimetic membrane technology significantly enhances drug enrichment at atherosclerotic plaques. Organ distribution studies showed both nanoparticle types accumulated in the liver, P@Ce6 NPs displayed notably stronger hepatic fluorescence than HA-M@P@Ce6 NPs (Fig. 6D). This discrepancy is likely attributed to the enhanced clearance of “non-stealth” nanoparticles by the hepatic reticuloendothelial system (RES) following systemic administration [42]. Surprisingly, the macrophage membrane (Møm) coating on HA-M@P@Ce6 NPs appears to mitigate hepatic RES uptake, thereby promoting preferential accumulation at plaque sites. as shown in Fig. 6E, under identical imaging parameters, liver fluorescence intensities in both P@Ce6 and HA-M@P@Ce6 groups far exceeded those in the aorta, a finding consistent with the known passive hepatic accumulation of nanoparticles after intravenous injection [18, 43]. Despite this hepatic tropism, HA-M@P@Ce6 NPs still exhibit superior aortic targeting efficiency compared to P@Ce6 NPs. Given the liver’s central role in systemic lipid metabolism and its intimate association with atherosclerosis, the potential of HA-M@P@Ce6 NPs to target both the aorta and hepatic tissues may open new therapeutic strategies for managing atherosclerotic disease.
Pharmacokinetics and targeting of HA-M@P@(Evol + Cur) NPs. (A&B) Fluorescence images and pharmacokinetic profiles of P@Ce6 and HA-M@P@Ce6 at different time points; (C) The ex vivo fluorescence images of aorta in atherosclerotic mice (n = 3); (D) Ex vivo fluorescence distribution images in the heart, liver, spleen, lungs, and kidneys of ApoE−/− mice; (E) Quantitative analysis of fluorescence intensity in ex vivo heart, liver, spleen, lungs, kidneys, and aorta from ApoE−/− mice. Data are shown as mean ± SD (n = 3). *P < 0.05, ***P < 0.0001
The efficacy of HA-M@P@(Evol + Cur) NPs in inhibiting plaque progression in ApoE−/− mice in vivo
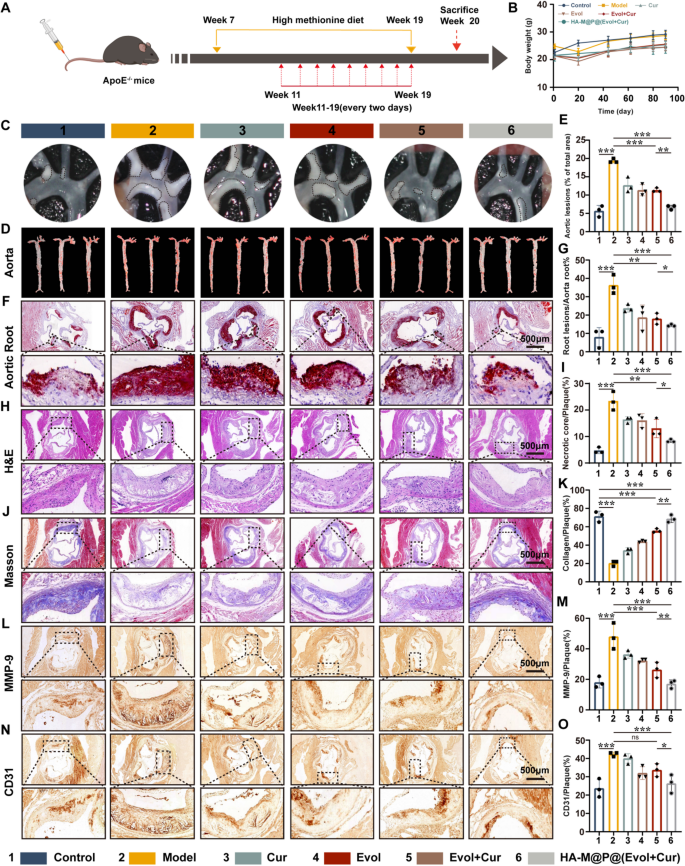
Based on the above in vitro results, we further evaluated the therapeutic effects of HA-M@P@(Evol + Cur) NPs on ApoE−/− atherosclerotic mice in vivo. The treatment protocol is shown in Fig. 7A. Ultrasonography images indicated significant thicken of the aortic root and acceleration of blood flow velocity in the model group compared to normal diet ApoE−/− mice. In turn, HA-M@P@(Evol + Cur) NPs treatment reduced the intima-media thickness and the blood flow velocity as well (Fig. S9A&B). Meanwhile, HA-M@P@(Evol + Cur) NPs treatment relatively reduced the body weight of mice, compared to the model group (Fig. 7B). By investigating the therapeutic effects of HA-M@P@(Evol + Cur) NPs on atherosclerotic plaques in vivo, we observed a significant reduction in the size of plaques in the aortic arch (area surrounded by the black dotted circle) (Fig. 7C). Additionally, the aortas of the mice were collected at the end of the treatment and stained for ORO, as shown in the Fig. 7D&E, the model group showed the largest ORO-positive area (approximately 19.14% of the area), while in contrast, the normal mice showed the smallest ORO-positive area (approximately 5.48% of the area), indicating the successful modeling of atherosclerosis. Furthermore, we studied the effects of HA-M@P@(Evol + Cur) NPs on plaque formation in high-incidence aortic roots. ORO staining of freeze-dried sections showed significant lipid deposition in plaques from the model group (approximately 36.49%), both Evol + Cur and HA-M@P@(Evol + Cur) NPs treatment reduced the plaque area. Especially, the average plaque area in ApoE−/− mice was reduced from 36.49 to 14.15% after treated with HA-M@P@(Evol + Cur) NPs (Fig. 7F&G). Additional histochemical studies of aortic sinus sections were further conducted. The images of H&E staining showed that numerous lipid-filled necrotic cores were observed in the model group, while these cores appeared marginally reduced in size in mice treated with Evol + Cur. Notably, there was a substantial reduction in the size of necrotic cores by approximately 15.2% in mice treated with HA-M@P@(Evol + Cur) NPs (Fig. 7H&I).
Plaque stability is crucial for patient outcomes, as studies have shown that 75% of coronary syndrome cases are caused by plaque rupture [44]. Collagen content, which is strongly associated with plaque stability, decreases as atherosclerosis progresses [45]. Plaque collagen content was measured using Masson’s trichrome staining. The fibrous cap thickness and collagen content rose considerably after HA-M@P@(Evol + Cur) NP treatment (Fig. 7J&K). It was reported that, matrix metalloproteinase-9 (MMP-9) is closely linked to plaque stability, predominantly localized in the shoulder region of atherosclerotic plaques, the necrotic core, and the fibrous cap [46]. Higher levels and activity of MMP-9 are observed in unstable plaques compared to stable ones. MMP-9 antibody staining showed that HA-M@P@(Evol + Cur) NPs therapy effectively reduced MMP-9 expression in plaques (Fig. 7L&M). Moreover, immunohistochemistry staining with anti-CD31 antibody (HUVECs marker) revealed that HA-M@P@(Evol + Cur) NPs effectively reduced the number of CD31+ new vessel in the atherosclerotic plaque region, reducing the vulnerable plaques caused by the large brittle rupture of the rich vascular network formed by neovascularization (Fig. 7N&O). Collectively, these data, taken as a whole, provide evidence that HA-M@P@(Evol + Cur) NPs have the potential to be therapeutically effective in reducing the progression of atherosclerosis while simultaneously increasing the stability of plaques.
The efficacy of HA-M@P@(Evol + Cur) NPs in inhibiting plaque progression in ApoE−/− mice in vivo. (A) Schematic diagram of treatment plan; (B) The impact of different treatment regimens on the body weight of ApoE−/− mice with atherosclerosis; (C) Photographs of the aortic arch; (D&E) Oil Red O (ORO) staining images and semi-quantitative analysis of aortas from AS model mice subjected to different treatments (n = 3); (F&G) Typical representative images and semi-quantitative analysis of ORO staining of the aortic root from AS model mice subjected to different treatments, Scale bar = 500 μm; (H–K) Representative images of H&E and Masson’s trichrome staining in the aortic root and semi-quantitative analysis, Scale bar = 500 μm (n = 3); (L–O) Representative histochemical images and semi-quantitative analysis of aortic root cross sections stained with MMP-9 antibody and CD31 antibody, Scale bar = 500 μm (n = 3). Data are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.001, ***P < 0.0001. ns, not significant
The ability of HA-M@P@(Evol + Cur) NPs to regulate cholesterol efflux and inhibit inflammatory response and in ApoE−/− mice
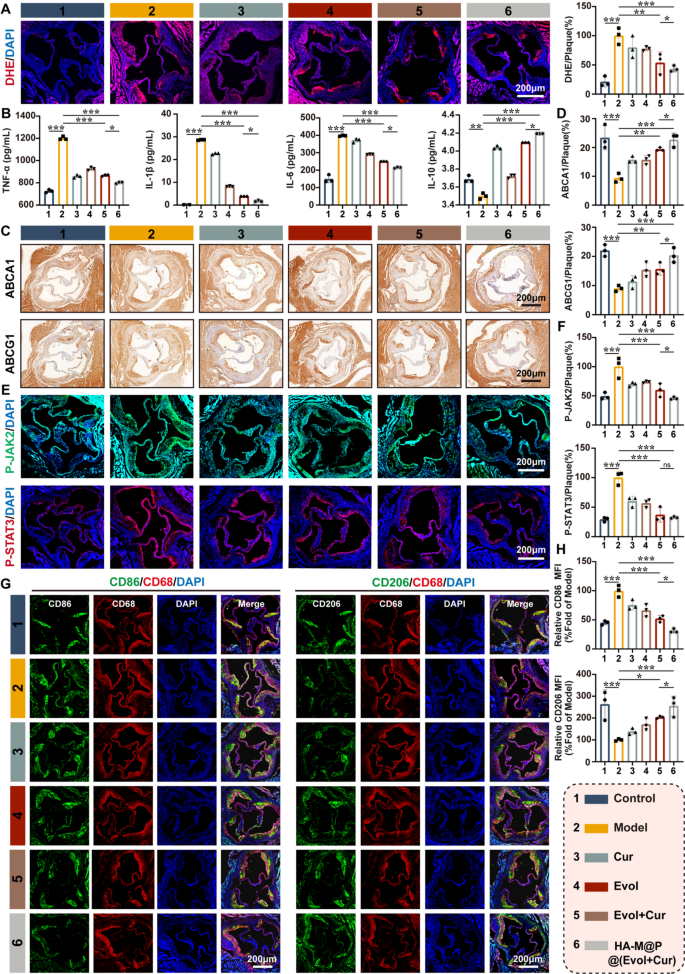
Previous studies have shown high levels of ROS in the atherosclerotic plaques, which is the basis for the pathogenesis of atherosclerosis [47]. As HA-M@P@(Evol + Cur) NPs can efficiently scavenge macrophages ROS in vitro, we naturally investigated the effect of ROS scavenge on the inflammatory response in vivo. At first, DHE staining of fresh frozen aortic roots from ApoE−/− mice indicated strong red fluorescent signal in the model group, which reflected the high ROS levels. In contrast, only weak red fluorescent signal appeared in the aortic roots from ApoE−/− mice treatment with HA-M@P@(Evol + Cur) NPs (Fig. 8A). Then, we detected the changes inflammatory factors in mice by ELISA and found the decrease in the levels of TNF-α, IL-1β, and IL-6 and an up-regulation of the levels of the anti-inflammatory factor IL-10 in the serum of mice with HA-M@P@(Evol + Cur) NPs treatment (Fig. 8B). High expression of proinflammatory factors has been reported to inhibit the levels of ABCA1 and ABCG1 in macrophages, which finally lead to lipid accumulation in plaques and aggravation of atherosclerosis [32, 48]. Immunohistochemical staining of paraffin sections of the aortic root showed that treatment with HA-M@P@(Evol + Cur) NPs increased the expression of ABCA1 and ABCG1 (Fig. 8C&D). Meanwhile, compared to the model group, the HA-M@P@(Evol + Cur) NPs group significantly downregulated the levels of p-JAK2 and p-STAT3 (2.1-fold and 3.1-fold reduction) (Fig. 8E&F). Furthermore, immunofluorescence staining indicated high expression of CD86 and low expression of CD206 in the aortic sections of high-homocysteine diet fed ApoE−/− mice, HA-M@P@(Evol + Cur) NPs treatment significantly altered the distribution of macrophage subpopulations within the plaques, with an increase in the number of CD206+ cells and a decrease in the number of CD86+ cells observed at the plaques (Fig. 8G&H). Together, these results demonstrated that HA-M@P@(Evol + Cur) NPs could shift the inflammatory response to an anti-inflammatory state via JAK2/STAT3 pathway, which ultimately resulted in the up-regulation of ABCA1 and ABCG1 to promote cholesterol efflux from macrophages at the plaques and slow down the progression of atherosclerosis.
The ability of HA-M@P@(Evol + Cur) NPs to regulate cholesterol efflux and inhibit inflammatory response in ApoE−/− mice. (A) Image and quantitative analysis of dihydroethidium staining on the transverse section of aortic root; (B) ELISA analysis of serum TNF-α, IL-1β, IL-6 and IL-10 levels in ApoE−/− mice; (C&D) Representative histochemical images of aortic root cross sections stained with ABCA1 antibody and ABCG1 antibody; (E&F) aortic root sections were subjected to anti-p-JAK2 (green) and anti-p-STAT3 (red) immunofluorescence staining images and quantitative analysis; (G&H) Co-immunofluorescence staining images and quantitative analysis of anti-CD86 (green) and CD68 (red) and anti-CD206 (green) and CD68 (red) in aortic root sections. All scale bars = 200 μm. Data are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.001, ***P < 0.0001. ns, not significant
HA-M@P@(Evol + Cur) NPs attenuate hcy-induced hepatic steatosis and modulate metabolites and intestinal flora
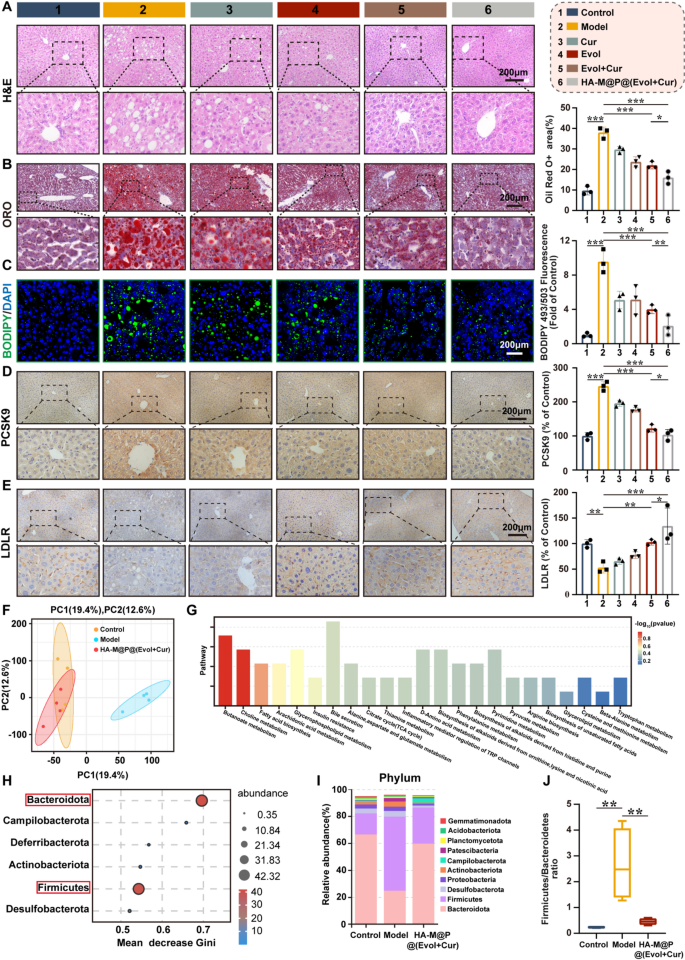
According to reports, elevated levels of Hcy may induce hepatic steatosis, which is closely associated with the risk of cardiovascular diseases and cardiovascular events [49]. To accurately assess the impact of HA-M@P@(Evol + Cur) NPs on the liver histopathology of ApoE−/− mice, liver samples were collected at the end of the experiment for histological analysis. Hematoxylin and eosin (H&E) staining revealed that, compared to the normal control group, the liver tissue sections of the model group exhibited numerous vacuole-like structures of varying sizes, indicating significant characteristics of hepatocellular steatosis. Notably, intervention with HA-M@P@(Evol + Cur) NPs significantly ameliorated this pathological alteration (Fig. 9A). To clearly identify cells containing lipid droplets and quantify their abundance, liver tissues were specifically stained with Oil Red O. The results obtained were consistent with those from H&E staining. Oil Red O staining demonstrated that treatment with HA-M@P@(Evol + Cur) NPs reduced lipid droplet deposition in hepatocytes by approximately 57% compared to the model group (Fig. 9B). Analogously, BODIPY fluorescence staining results showed a significant increase in lipid droplet content in the hepatocytes of high-homocysteine diet fed induced ApoE−/− mice, as indicated by strong green fluorescence signals. Following treatment with HA-M@P@(Evol + Cur) NPs, the green fluorescence intensity reflecting lipid droplet content was markedly reduced, suggesting that this nano-formulation effectively inhibits lipid accumulation in liver tissue (Fig. 9C). Quantitative analysis further confirmed that the liver triglyceride (TG) and total cholesterol (TC) levels in mice treated with HA-M@P@(Evol + Cur) NPs were reduced by 30% and 52%, respectively, compared to the model group (Fig. S10A&B). These results indicate that HA-M@P@(Evol + Cur) NPs may provide potential benefits in alleviating Hcy-induced hepatic steatosis.
To investigate the molecular mechanisms by which HA-M@P@(Evol + Cur) NPs ameliorate Hcy-induced hepatic steatosis, this study employed immunohistochemical methods to assess the expression levels of key regulatory factors involved in hepatic lipid metabolism. LDLR plays a central role in promoting the clearance of circulating LDL and the feedback inhibition of cholesterol biosynthesis, serving as a critical regulator of plasma cholesterol homeostasis. In contrast, proprotein convertase subtilisin/kexin type 9 (PCSK9) increases plasma LDL-C levels by accelerating LDLR degradation [50]. Consistent with previous reports, our results demonstrated that, compared to the normal control group, the expression of PCSK9 in the liver of the model group was significantly upregulated, accompanied by downregulated LDLR expression. Notably, intervention with HA-M@P@(Evol + Cur) NPs exhibited a stronger regulatory effect than the combination of Evol and Cur alone, significantly suppressing PCSK9 expression while promoting LDLR expression (Fig. 9D&E). The export of cholesterol from the liver is essential for maintaining hepatic lipid metabolism homeostasis, with ABCA1 and ABCG1 being key proteins mediating this process [51]. Immunohistochemical analysis revealed that, compared to the model group, the expression levels of ABCA1 and ABCG1 in the liver tissues of the HA-M@P@(Evol + Cur) NPs-treated group were significantly elevated (Fig. S11A&B). These findings suggest that HA-M@P@(Evol + Cur) NPs may improve lipid metabolism disorders through a dual mechanism: on one hand, enhancing the liver’s capacity to uptake circulating cholesterol, and on the other hand, promoting hepatic lipid efflux. This effectively alleviates Hcy-induced hepatic steatosis and provides a potential therapeutic strategy for delaying the progression of atherosclerosis.
To further elucidate the protective mechanisms of HA-M@P@(Evol + Cur) NPs against Hcy-induced hepatic steatosis, we employed serum metabolomics and 16 S rRNA gene sequencing to systematically analyze serum metabolites and fecal metabolic profiles in ApoE−/− mice across control, model, and HA-M@P@(Evol + Cur) NPs intervention groups. Principal component analysis (PCA) of metabolomics data revealed significant differences in metabolites among the three groups (Fig. 9F). Venn diagram analysis identified 161 common differential metabolites between the control, model, and HA-M@P@(Evol + Cur) NPs intervention groups (Fig. S12A). Volcano plot analysis further demonstrated that 62/145 compounds were up-/down-regulated in the control group compared to the model group. Similarly, 157/71 compounds were up-/down-regulated in the model group compared to the HA-M@P@(Evol + Cur) NPs group (Fig. S12B). These significant differential metabolites induced by HA-M@P@(Evol + Cur) NPs intervention were primarily associated with 23 metabolic pathways, including butyrate metabolism, choline metabolism, fatty acid synthesis, arachidonic acid metabolism, glycerophospholipid metabolism, bile secretion, and the tricarboxylic acid (TCA) cycle (Fig. 9G). These pathways are closely linked to the development and progression of atherosclerosis (AS) and are crucial for understanding its pathogenesis [52]. Further analysis revealed that key metabolites in these pathways are closely related to hepatic lipid metabolism disorders. Studies have shown that abnormal changes in glycerophosphorylcholine and β-hydroxybutyrate levels can exacerbate the progression of atherosclerosis [53]. Meanwhile, arachidonic acid, as a critical inflammatory mediator, participates in pathological changes in the arterial wall through multiple signaling pathways [54]. Notably, patients with hyperhomocysteinemia often exhibit significant inhibition of arachidonic acid metabolism [55]. Additionally, Saijou et al. found that choline maintains lipid metabolism balance by promoting hepatic cholesterol clearance and lipoprotein uptake, while choline deficiency leads to abnormal accumulation of fatty acids and cholesterol in the liver [56]. This study demonstrated that HA-M@P@(Evol + Cur) NPs significantly upregulate the levels of glycerophosphocholine, arachidonate, choline, indole-3-acetic acid, pyridoxal phosphate, and citrulline, while downregulating the expression of β-hydroxybutyrate and lysophosphatidylcholine (Fig. S13). These alterations in metabolites are associated with multiple pathological processes of AS: indole-3-acetic acid exhibits anti-inflammatory and antioxidant effects, regulating metabolic homeostasis; deficiencies in vitamin B6 (pyridoxal phosphate) and citrulline exacerbate Hcy-induced AS [57, 58]; and lysophosphatidylcholine, as an atherogenic lipoprotein component, promotes tissue inflammation by releasing fatty acids and arachidonic acid derivatives [57]. In summary, this study reveals, from a metabolomics perspective, the potential mechanisms by which HA-M@P@(Evol + Cur) NPs delay AS progression through the regulation of hepatic lipid metabolism.
The gut microbiota is a highly complex and diverse ecosystem that plays a critical role in metabolic regulation and immune defense in the host [59]. Previous studies have shown that alterations in the structure of the gut microbiota can participate in the pathological process of AS by influencing hepatic lipid metabolism [60, 61]. At the end of the experiment, we collected fecal samples from mice and performed 16 S rRNA sequencing to thoroughly analyze changes in the composition of the gut microbiota. The results revealed that, compared to the control group, the number of gut microbiota in the model group increased, while treatment with HA-M@P@(Evol + Cur) NPs reduced the quantity of gut microbiota (Fig. S14A). The Shannon and Simpson indices were used to assess species richness and diversity, respectively. As shown in Fig. S14B&C, the richness and diversity of the gut microbiota were significantly reduced in ApoE−/− fed a high-homocysteine diet, while HA-M@P@(Evol + Cur) NPs treatment markedly improved this condition. This improvement may be attributed to the alteration in the abundance of specific microbial populations induced by HA-M@P@(Evol + Cur) NPs, leading to differences in species richness among the three groups [62]. Principal component analysis (PCA) and non-metric multidimensional scaling (NMDS) demonstrated significant differences in microbial community structure among the three groups (Fig. S14D&E). At the phylum level, random forest analysis identified Bacteroidetes and Firmicutes as the key species contributing to the differences between the two groups (Fig. 9H). Notably, patients with atherosclerosis often exhibit gut dysbiosis, characterized by an increased Firmicutes/Bacteroidetes ratio [63]. This study found that a high-homocysteine diet not only reduced the relative abundance of Bacteroidetes but also increased the proportion of Firmicutes, thereby elevating the Firmicutes/Bacteroidetes ratio. However, HA-M@P@(Evol + Cur) NPs intervention effectively reversed this change (Fig. 9I&J). Linear discriminant analysis (LDA) and linear discriminant analysis effect size (LEfSe) analysis confirmed that HA-M@P@(Evol + Cur) NPs could modulate AS-related beneficial microbiota at the phylum level (Fig. S15A&B). At the genus level, analysis revealed a significant increase in harmful genera (e.g., Ileibacterium, Desulfovibrio, Allobaculum, Lactobacillus, and Dubosiella) and a marked decrease in beneficial genera (e.g., Helicobacterium, Muribaculum, Bacteroides, and Lachnospiraceae_NK4A136_) in the model group. Treatment with HA-M@P@(Evol + Cur) NPs significantly reversed these changes in microbial abundance (Fig. S16A). A ternary phase diagram further corroborated this finding (Fig. S16B). Heatmaps of bacterial population distribution and Circos analysis at the species level further confirmed significant differences in community composition between the HA-M@P@(Evol + Cur) NPs intervention group and the model group (Fig. S17A&B). In summary, the findings of this study demonstrate that HA-M@P@(Evol + Cur) NPs can modulate the structure of the gut microbiota, providing new evidence for their role in regulating hepatic lipid metabolism and ultimately exerting anti-atherosclerotic effects.
HA-M@P@(Evol + Cur) NPs attenuate Hcy-induced hepatic steatosis and modulate metabolites and intestinal flora. (A) Representative H&E staining image of liver tissue; (B) Representative oil red O staining image of liver tissue and quantitative analysis; (C) Representative BODIPY staining image of liver tissue and quantitative analysis. (D) Immunohistochemical staining images and semi-quantification of PCSK9 in the liver tissue of ApoE−/− mice after different treatments; (E) Immunohistochemical staining images and semi-quantification of LDLR in the liver tissue of ApoE−/− mice after different treatments; All scale bars = 200 μm. (F) PCA principal component plots showing the differences between control, model and HA-M@P@(Evol + Cur) groups; (G) Differential metabolic pathways present between control, model and HA-M@P@(Evol + Cur) groups; (H) Random forest distribution calculating the contribution of each strain to the difference between the model and HA-M@P@(Evol + Cur) groups; (I) Histograms of relative abundance at the phylum level between the control, model, and HA-M@P@(Evol + Cur) groups; (J) Ratio of Firmicutes/Bacteroidetes phylum; Data are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.001, ***P < 0.0001
The biocompatibility and biosafety of HA-M@P@(Evol + Cur) NPs
Biocompatibility is crucial for determining the possibility of nanomaterials for clinical applications [64]. MTT assay was used to detect the cytotoxicity of P@(Evol + Cur) NPs, HA-M@P@(Evol + Cur) NPs on different cells. After co-culture with 220 µg/mL NPs, it showed different degrees of pro-proliferative effects in RAW264.7 and HepG2, and more than 80% viability in HUVEC, VSMC, H9C2 and HK-2 (Fig. S18A-F). After incubation with blood samples for 4 h, the hemolytic effect of NPs was negligible even at the highest concentration of 220 µg/mL NPs (Fig. S18G&H). In addition, erythrocytes incubated with NPs still showed intact morphology (Fig. S18I). The foregoing data show that NPs exhibit low cytotoxicity and favorable biocompatibility in vitro.
Nanomedicine biosafety is often assessed using zebrafish embryos due to their simple reproductive mechanism, quick developmental cycle, and great genetic similarity to human genes [65]. By analyzing these parameters in zebrafish, the effect of NPs on developmental characteristics was assessed. This study examined the effects of drugs including HA-M@P@(Evol + Cur) NPs on the developmental stages of zebrafish. The data showed that there were no significant differences in hatching process morphology, survival, and pre-hatching embryonic heartbeat between Evol + Cur, P@(Evol + Cur) NPs and HA-M@P@(Evol + Cur) NPs groups after hatching (Fig. S19A-C). These findings suggest that HA-M@P@(Evol + Cur) NPs have high biosafety, which is urgent for in vivo AS treatment.
In vivo, biosafety evaluations showed that both routine blood indices and clinical serum biochemical parameters in ApoE−/− mice treated with HA-M@P@(Evol + Cur) NPs were shown to be out of the normal range (Fig. S20A-G). In addition, the levels of LDL, HDL, TC and TG were most highly comparable (Fig. S20H-K). Compared with the high level of Hcy in the model group, serum Hcy in ApoE−/− mice treated with HA-M@P@(Evol + Cur) NPs was reduced to normal (Fig. S20L). H&E staining of the heart, spleen, lungs, and kidneys confirmed that in vivo injection of NPs did not damage these major organs, including morphological changes or signs of inflammation (Fig. S20M), which showed trapezoidal, well-organized myocardial fibers, red-white bone marrow of the spleen, well-defined alveolar structures, and uniformly sized and shaped glomeruli and well-defined renal borders. The above results indicate that HA-M@P@(Evol + Cur) NPs have high biological safety and potential for clinical application.