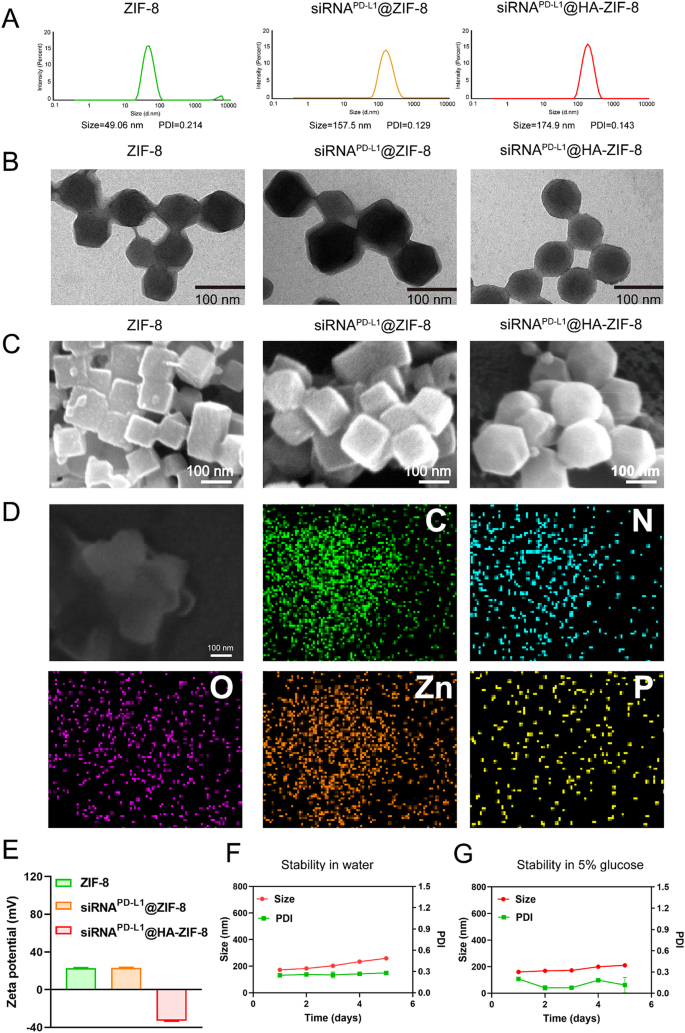
Preparation and characterization of siRNAPD−L1@HA-ZIF-8
ZIF-8 was obtained by a simple one-pot method, in which equal volumes of different concentrations of 2-methyl imidazole and zinc nitrate hexahydrate were mixed for self-assembly. To obtain ZIF-8 nanoparticles loaded with siRNAPD−L1, siRNAPD−L1 with different mass ratios to ZIF-8 was premixed with 2-methyl imidazole solution and then added with zinc nitrate hexahydrate solution. The mixture was vortexed to obtain siRNAPD−L1@ZIF-8 NPs. When the mass ratio of ZIF-8 to siRNAPD−L1 was 500, the obtained siRNAPD−L1@ZIF-8 NPs had the most optimal particle size (Fig. S1). Subsequently, the HA solution was incubated with siRNAPD−L1@ZIF-8 NPs to obtain siRNAPD−L1@HA-ZIF-8 NPs by modifying on the surface through electrostatic interactions, with simple preparations. The particle sizes of ZIF-8, siRNAPD−L1@ZIF-8 and siRNAPD−L1@HA-ZIF-8 NPs were 49.06 nm, 157.5 nm and 174.9 nm, respectively (Fig. 1A). After encapsulating siRNAPD−L1 and covering HA, the particle sizes of the nanoparticles both increased in different degrees respectively, while still remaining within the appropriate levels. In addition, the surface potential of ZIF-8 showed a strong positive charge (23.5 mV), whereas the surface potential experienced no significant change and remained positive (22.6 mV) after the encapsulation of siRNAPD−L1 (Fig. 1E). It could be that the negative charge of siRNAPD−L1 was too weak to neutralize the strong positive charge of ZIF-8 or the siRNAPD−L1 was encapsulated inside ZIF-8 and therefore had little effect on the surface potential. In contrast, after coating HA, the surface potential of siRNAPD−L1@HA-ZIF-8 NPs displayed a strong negative charge (-32.8 mV), favoring stability in the blood circulation with a prolonged circulation time. Subsequently, the structure of ZIF-8 and siRNAPD−L1@ZIF-8 NPs exhibited the rhombic dodecahedron with sharp edges and clear corners, as shown in transmission electron microscopy (TEM). After covering HA, siRNAPD−L1@HA-ZIF-8 NPs displayed a core-shell structure and smooth edges (Fig. 1B). Similar results were also presented in the surface morphology photographed by scanning electron microscopy (SEM), where ZIF-8 and siRNAPD−L1@ZIF-8 NPs exhibited polyhedral shapes, whereas siRNAPD−L1@HA-ZIF-8 NPs displayed a spherical shape (Fig. 1C). The elemental mapping displayed the spatial distribution of C, N, O, Zn and P elements in the siRNAPD−L1@HA-ZIF-8 NPs, where the uniform distribution of Zn elements indicated the successful preparation of ZIF-8 and the distribution of P elements indicated the effective encapsulation of siRNAPD−L1 into ZIF-8 (Fig. 1D). All the above results proved the successful preparation of siRNAPD−L1@HA-ZIF-8 NPs.
Much literature has reported that ZIF-8 has pH-sensitive degradation properties [49, 50]. Therefore, the pH-sensitive degradation properties of the nanoparticles were confirmed by TEM and DLS analysis. After adding pH 5.5 medium, the morphology of siRNAPD−L1@HA-ZIF-8 NPs became blurred and the specific structure could not be visible from the TEM images (Fig. S2A), indicating that the nanoparticles had been cleaved and destroyed. Meanwhile, DLS analysis demonstrated that siRNAPD−L1@HA-ZIF-8 NPs had a much larger particle size of more than 2000 nm after incubating with the medium at pH 5.5 (Fig. S2B). These findings confirmed the pH-sensitive cleavage and destruction properties of siRNAPD−L1@HA-ZIF-8 NPs. Additionally, a Zn2+ ion release experiment was conducted to further demonstrate the pH-sensitive degradation properties of siRNAPD−L1@HA-ZIF-8 NPs. Under normal physiological conditions (pH 7.4), approximately 25% of Zn2+ was released within 24 h. In contrast, under simulated tumor lysosome conditions (pH 5.5), the release of Zn2+ was significantly higher, reaching approximately 78% (Figure S3). Under acidic conditions, protonation of the organic ligand disrupts the Zn-imidazolate coordination bonds, resulting in the decomposition of the ZIF-8 framework and subsequent release of Zn2+ [51]. It suggests that siRNAPD−L1@HA-ZIF-8 NPs could degrade under acidic stimuli but remain stable under physiological conditions. Moreover, we examined the storage stability of siRNAPD−L1@HA-ZIF-8 NPs in water and 5% glucose. As can be seen from Fig. 1F and G, there was no significant change in the particle size and PDI of siRNAPD−L1@HA-ZIF-8 NPs in water and 5% glucose over 5 days, indicating that the nanoparticles have excellent storage stability for subsequent experiments.
Characterization of siRNAPD−L1@HA-ZIF-8 NPs. (A) Hydrodynamic particle size of ZIF-8, siRNAPD−L1@ZIF-8 and siRNAPD−L1@HA-ZIF-8 NPs measured by dynamic light scattering (DLS). (B) TEM images of ZIF-8, siRNAPD−L1@ZIF-8 and siRNAPD−L1@HA-ZIF-8 NPs in pH 7.4 medium. (C) SEM images of ZIF-8, siRNAPD−L1@ZIF-8 and siRNAPD−L1@HA-ZIF-8 NPs. (D) Element mappings of siRNAPD−L1@HA-ZIF-8 NPs. (E) Zeta potential of ZIF-8, siRNAPD−L1@ZIF-8 and siRNAPD−L1@HA-ZIF-8 NPs. The particle size change of siRNAPD−L1@HA-ZIF-8 NPs stored in water (F) and 5% glucose (G). Results were presented as mean ± SD
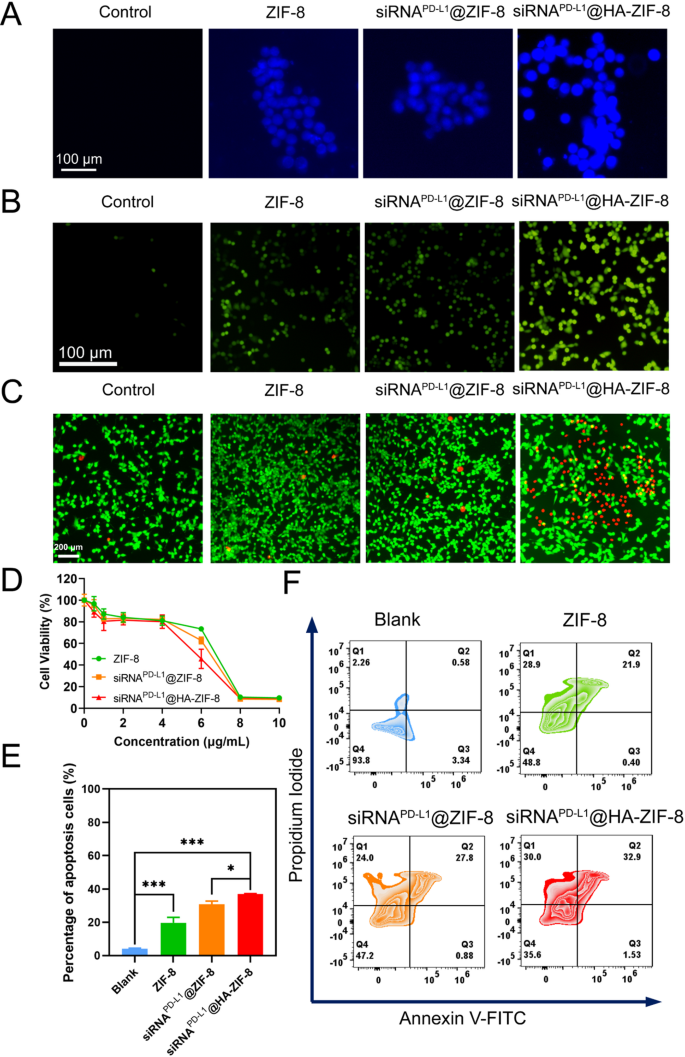
In vitro antitumor efficacy
A key factor for nanoparticles to exert therapeutic effects is efficient cellular uptake, so we investigated the uptake efficiency of FITC-labelled siRNAPD−L1@HA-ZIF-8 NPs by 4T1 cells. The intensity of the fluorescent signal of FITC gradually increased with the prolonged incubation time, indicating that siRNAPD−L1@HA-ZIF-8 NPs could be effectively internalized by 4T1 cells due to the binding of HA to the CD44 receptor on the cell surface (Fig. S4). In addition, Zn2+ ions were the critical elements for the prepared nanoparticles to exert anti-tumor efficacy, so the Zn2+ ion probe N-(6-Methoxy-8-quinolyl)-p-toluenesulfonamide (TSQ) was adopted to detect the intracellular Zn2+ ions. As expected, intracellular Zn2+ ions in the nanoparticle group were dramatically higher than that in the Control group, with the highest intracellular Zn2+ levels in siRNAPD−L1@HA-ZIF-8 group (Fig. 2A), which was attributed to higher intracellular internalization by 4T1 cells due to modification of HA. Also, the quantification of intracellular Zn²⁺ levels in 4T1 cells following incubation with various formulations corroborated the same results, as determined by a Zinc Colorimetric Assay Kit (Fig. S5). Zn2+ ions in the tumor cells could induce the production of reactive oxygen species (ROS), promoting cell death [52, 53]. Consequently, we utilized a ROS probe, 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) to validate intracellular ROS production. Compared with the Control group, 4T1 cells treated with nanoparticles all produced obvious ROS fluorescence signals, with the most intense in siRNAPD−L1@HA-ZIF-8 group (Fig. 2B), which may be attributed to the high amount of Zn2+ ions after the treatment of siRNAPD−L1@HA-ZIF-8 NPs.
High concentrations of ROS could cause toxicity to tumor cells, so CCK-8 assays were performed to investigate the proliferation inhibitory of the nanoparticles on 4T1 cells. The survival rates of the cells were all more than 80% when the concentration of Zn2+ ions was lower than 4 µg/mL (Fig. 2D), which is consistent with the property of ZIF-8 as a highly biocompatible material for drug delivery. However, when the concentration of Zn2+ ions continued to increase, the cell survival rate decreased abruptly, with the most obvious downward trend in the siRNAPD−L1@HA-ZIF-8 NPs group. This phenomenon may result from the instantaneous disruption of osmotic balance and generation of ROS due to the sustained release of Zn2+ ions. Also, we performed living and dead cell staining assay by Calcein-AM and propidium iodide (PI). As can be seen from Fig. 2C, there were barely red fluorescence signals (dead cells) in the Control group, while there was a little presence in the ZIF-8 and siRNAPD−L1@ZIF-8 groups. In contrast, 4T1 cells treated with siRNAPD−L1@HA-ZIF-8 NPs displayed massive red fluorescent signals, indicating the presence of abundant dead cells, which was caused by the high concentration of ROS. To quantify the effect of nanoparticles in inducing cell death, we used Annexin V-FITC and propidium iodide (PI) to stain the cells for flow cytometry apoptosis assay. Compared with the Control group, ZIF-8, siRNAPD−L1@ZIF-8 and siRNAPD−L1@HA-ZIF-8 NPs all induced obvious apoptosis, with apoptotic cell percentages of 22.3%, 28.7% and 34.4%, respectively (Fig. 2E). Simultaneously, high rates of necrosis were observed in these three groups, up to 28.9%, 24% and 30%, respectively (Fig. 2F). Therefore, the above results suggested that the mechanisms of cell death induced by nanoparticles may be more than apoptosis, but also other mechanisms of cytocidal action.
In vitro cytotoxicity studies. (A) Intracellular Zn2+ ions detection by TSQ. (B) Intracellular ROS levels. (C) Live/dead cell staining of 4T1 cells after various treatments. Green: living cells. Red: dead cells. (D) Viability of 4T1 cells after various treatments. (E) Quantitative analysis of cell apoptosis. (F) Flow cytometry apoptosis assay with different treatments. Data were presented as mean ± SD, *P < 0.05, ***P < 0.001
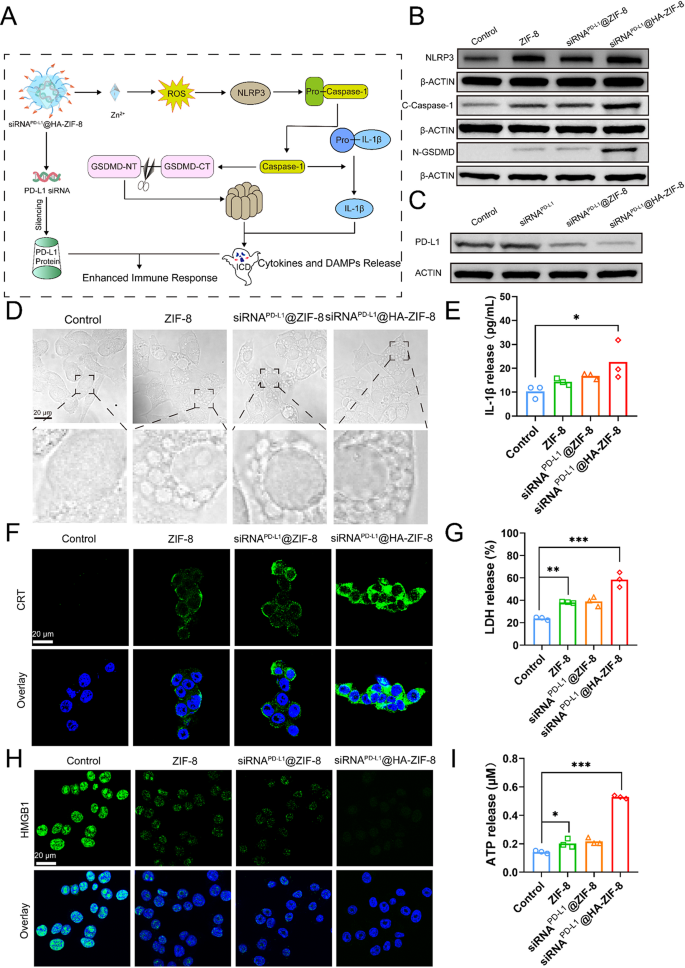
Activation of pyroptosis and ICD effect
As widely known, pyroptosis is a distinct form of programmed cell death characterized by cell swelling and the appearance of large bubbles. Thus, the morphology of necrotic cells was observed to determine whether the nanoparticles induced pyroptosis. In the microscope images, 4T1 cells treated with ZIF-8, siRNAPD−L1@ZIF-8 and siRNAPD−L1@HA-ZIF-8 NPs exhibited swelling, bubbling, and eventually bursting with increasing incubation time (Fig. S6), which were remarkable features of pyroptosis. What’s more, it appeared that a great number of vesicles emerged inside the cells treated with nanoparticles in the high magnification images (Fig. 3D), which could be pyroptotic bodies [54], also known as inflammasome, formed before the rupture of the cell membrane when pyroptosis occurred. Accordingly, it is reasonable to speculate that ROS induced by nanoparticles through releasing Zn2+ ions could activate NLRP3 inflammasome. Subsequently, pyroptosis is triggered via the classical Caspase-1-dependent pathway, which ultimately triggers ICD and the release of cytokines and DAMPs to evoke anti-tumor immune activation (Fig. 3A).
To elucidate the mechanism of Zn2+ overload-induced pyroptosis, we examined mitochondrial integrity, as mitochondria are essential for maintaining cellular energy homeostasis and are particularly vulnerable to damage from excess Zn2+ [55]. The JC-1 fluorescent dye was employed to measure alterations in mitochondrial membrane potential, a key parameter that reflected mitochondrial functional integrity. After treatment with nanoparticles, tumor cells exhibited enhanced JC-1 monomer fluorescence and attenuated J-aggregate fluorescence (Fig. S7), demonstrating that excessive Zn2+ caused significant mitochondrial damage. Mitochondrial damage induces the formation and activation of NLRP3 inflammasomes, subsequently activating the Caspase-1-dependent pyroptosis pathway. In the classical Caspase-1-dependent pyroptosis pathway, activated Caspase-1 cleaves Gasdermin D (GSDMD) to form a peptide containing the active domain of the nitrogen terminus of Gasdermin D (N-GSDMD), which induces perforation of the cell membrane, leading to cellular rupture and release of the contents, ultimately inducing the inflammatory reaction. Meanwhile, activated Caspase-1 cleaves the precursor of IL-1β to form active IL-1β and releases it extracellularly, thereby recruiting inflammatory cells and amplifying the inflammatory reaction [56]. Western blot assays revealed that all three nanoparticle groups exhibited significantly increased levels of NLRP3 inflammasomes, cleaved Caspase-1 (C-Caspase-1) and N-GSDMD compared to the Control group, with the highest in the siRNAPD−L1@HA-ZIF-8 NPs group (Fig. 3B). Cell membrane pores and even cell membrane rupture derived from pyroptosis could potentially cause the release of associated inflammatory molecules and contents, including IL-1β and LDH [57]. As shown in Fig. 3E and G, the siRNAPD−L1@HA-ZIF-8 NPs group could significantly stimulate the release of IL-1β and LDH, which were elevated 2-fold and 3-fold in the culture medium, respectively, compared with the Control group. As concluded, siRNAPD−L1@HA-ZIF-8 NPs induced a strong pyroptosis in tumor cells through the classical Caspase-1-dependent pathway.
Pyroptosis may elicit the release of DAMPs molecules, including calreticulin (CRT) exposure, adenosine triphosphate (ATP), and high-mobility group box-1 (HMGB1). As shown from the CLSM images after different treatments, more CRT exposure (green fluorescence) was observed in the cell membranes treated with the nanoparticle group, especially in the siRNAPD−L1@HA-ZIF-8 NPs group (Fig. 3F). The results of quantitative analysis of fluorescence intensity more visually demonstrated the dramatic effect in CRT exposure between the siRNAPD−L1@HA-ZIF-8 NPs and Control groups, with an increase of up to 30-fold (Fig. S8). Furthermore, an increased release of ATP (approximately 3-fold) was detected in the culture medium of siRNAPD−L1@HA-ZIF-8-treated cells compared to the Control group (Fig. 3I), further demonstrating that cells undergoing pyroptosis potentially had high immunogenicity. The released ATP acts as a “find me” signal and the exposed CRT delivers an “eat me” signal, both of which collaboratively recruit phagocytes and initiate an immune response [58]. The extracellular released HMGB1 is an important immunomodulator that promotes DC maturation, further facilitating T cell activation and antigen presentation. As shown in Fig. 3H, the Control group displayed strong green fluorescence overlapping with the nucleus, which was diminished in the ZIF-8 and siRNAPD−L1@ZIF-8 NPs groups, and the green fluorescence signal of HMGB1 was even almost invisible in the siRNAPD−L1@HA-ZIF-8 NPs group. Similar results were more intuitively reflected in the quantitative analysis of HMGB1 fluorescent signals (Fig. S9). Pyroptosis-induced ICD promotes massive release of DAMPs and TAAs from tumor cells, driving the maturation of DC to enhance antigen presentation and stimulate T cells. To validate pyroptosis-induced DC maturation, 4T1 tumor cells were pretreated with different preparations, and the supernatants were collected to incubate with bone marrow-derived DCs (BMDCs). Notably, tumor supernatants pretreated with nanoparticles exhibited superior efficacy in promoting DC maturation (Fig. S10). Compared to Control group, siRNAPD−L1@HA-ZIF-8 NPs increased the proportion of mature DCs to 36.9%. Moreover, Zn2+ has been reported to activate the cGAS-STING pathway to promote DC maturation [59]. The WB analysis of DC cells treated with different preparations demonstrated that the phosphor STING (p-STING), phospho-IRF3 (p-IRF3) and phospho-TBK1 (p-TBK1) were obviously activated, indicating that siRNAPD−L1@HA-ZIF-8 NPs effectively activated the pathway to promote DC maturation (Fig. S11). Taken together, siRNAPD−L1@HA-ZIF-8 NPs can synergistically promote DC maturation by inducing tumor cell pyroptosis to release DAMPs and TAAs and activating the cGAS-STING pathway via Zn2+ release.
Aiming to evaluate the silencing efficiency of PD-L1 protein on the surface of tumor cells by nanoparticles, western blot assays were performed to detect the content of PD-L1 protein in 4T1 cells treated with free siRNAPD−L1, siRNAPD−L1@ZIF-8 and siRNAPD−L1@HA-ZIF-8 NPs. Compared with the Control and free siRNAPD−L1 groups, the PD-L1 protein content on the surface of 4T1 cells treated with siRNAPD−L1@ZIF-8 and siRNAPD−L1@HA-ZIF-8 NPs were significantly decreased, implying much more efficient silencing of PD-L1 proteins, with the highest silencing efficiency in the siRNAPD−L1@HA-ZIF-8 NPs group (Fig. 3C and Fig. S12). From the above results, it can be concluded that siRNAPD−L1@HA-ZIF-8 NPs triggered pyroptosis and ICD by the released Zn2+ ions, which resulted in the release of DAMPs and inflammatory factors, ultimately promoting DC maturation and activating T-cell activation. Meanwhile, siRNAPD−L1@HA-ZIF-8 NPs lead to the silencing of PD-L1 protein on the cell surface and inhibit tumor immune evasion. It is expected for siRNAPD−L1@HA-ZIF-8 NPs to synergistically enhance anti-tumor immunotherapy from the above two mechanisms (Fig. 3A).
Activation of pyroptosis and ICD Effect (A) Schematic illustration of the possible mechanism of siRNAPD−L1@HA-ZIF-8 NPs in promoting pyroptosis and silencing PD-L1 protein. (B) Western Blot analysis of NLRP3, C-Caspase-1 and N-GSDMD levels after various treatments. (C) Western Blot analysis of PD-L1 after various treatments. (D) Bright-field images of 4T1 cells by CLSM after different treatments. (E) The release of IL-1β after different treatments. (F) The fluorescence image of CRT exposure. (G) The release of LDH after different treatments. (H) The fluorescence image of HMGB1 immunofluorescence staining. (I) The release of ATP after different treatments. Data were shown as the means ± SD, *P < 0.05, **P < 0.01, ***P < 0.001
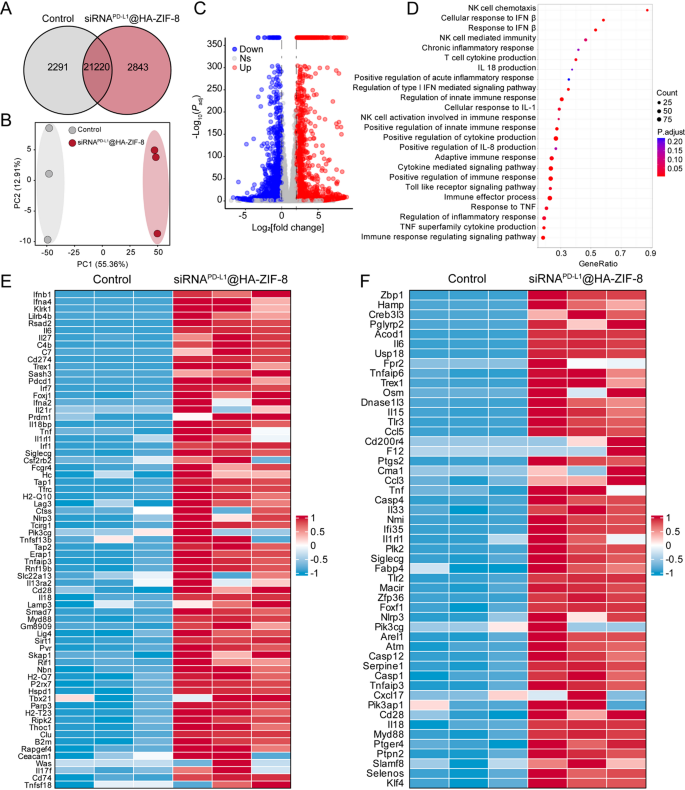
RNA sequencing analysis
To further investigate the mechanism of pyroptosis and related immune responses induced by siRNAPD−L1@HA-ZIF-8 NPs, we assessed the gene expression in 4T1 cells by RNA-seq after treatments of Control and siRNAPD−L1@HA-ZIF-8 NPs. The Venn diagram shows all the genes expressed in the Control and siRNAPD−L1@HA-ZIF-8 NPs groups (Fig. 4A). Principal component analysis (PCA) exposed significant differences between the two groups (Fig. 4B). As shown in the volcano plot (Fig. 4C), compared with Control group, 4034 differential genes were detected in siRNAPD−L1@HA-ZIF-8 NPs group, with 1842 genes up-regulated (red dots) and 2192 genes down-regulated (blue dots). Gene ontology (GO) enrichment analysis revealed that after siRNAPD−L1@HA-ZIF-8 NPs treatment, the differential genes were mainly enriched in inflammatory signaling pathways, pyroptosis signaling pathways and immune response-related signaling pathways, such as IFN signaling pathway, TNF signaling pathway, IL-18 production, Toll-like receptor signaling pathway and innate and adaptive immune response (Fig. 4D). Subsequently, we analyzed the expression levels of genes associated with the immune system and found that genes associated with adaptive immune response (Fig. 4E), innate immune response (Fig. S13) and natural killer cell-mediated immunity (Fig. S14) were significantly upregulated in siRNAPD−L1@HA-ZIF-8 NPs group. Meanwhile, similar up-regulations were also found in the expression levels of genes associated with pyroptosis, including genes involved in the inflammatory response (Fig. 4F), acute inflammatory response (Fig. S15) and tumor necrosis factor (Fig. S16). Furthermore, protein-protein interaction (PPI) networks demonstrated the co-expression and interaction of genes related to pyroptosis, inflammatory response and immune response (Fig. S17). In summary, siRNAPD−L1@HA-ZIF-8 NPs can effectively trigger pyroptosis, and promote inflammatory responses and cytokine release, thereby enhancing 4T1 tumor immunogenicity and provoking a strong anti-tumor immune response.
RNA sequencing analysis (A) Venn diagram of all expressed genes. (B) Principal component analysis diagram of each group. (C) Volcanic plot of differentially expressed genes between Control and siRNAPD−L1@HA-ZIF-8 NPs groups. (D) GO enrichment after siRNAPD−L1@HA-ZIF-8 NPs treatment. Heat map analysis of genes involved in adaptive immune response (E) and inflammatory response (F)
In vivo biodistribution and anti-tumor efficacy
In vitro experiments have demonstrated the strong pyroptosis and ICD effects induced by siRNAPD−L1@HA-ZIF-8 NPs, which encouraged us to explore their anti-tumor effects in vivo. The crucial issue for achieving therapeutic efficacy lies in access to the desired tissues and internalization by target cells. As proven in cellular uptake assays that siRNAPD−L1@HA-ZIF-8 NPs could be efficiently endocytosed by 4T1 cells, we subsequently investigated whether the nanoparticles could reach the tumor tissue using an in vivo imaging system. To intuitively observe the biodistribution in vivo, we took Cy5-labelled siRNA to prepare the nanoparticles. As shown in Fig. S18A, free siRNACy5 failed to enrich in the tumor and was rapidly cleared in vivo at 24 h post-injection. siRNACy5@ZIF-8 NPs displayed a limited amount of aggregation in the tumor. In contrast, siRNACy5@HA-ZIF-8 NPs presented better aggregation at the tumor site, and a significant level of fluorescent signal was still observed in the tumor even at 24 h post-injection. Afterwards, at 24 h post-injection, the heart, liver, spleen, kidney, lung and tumors of mice were dissected for ex vivo fluorescence imaging. Compared with free siRNACy5 and siRNACy5@ZIF-8, the fluorescence signal in tumors of siRNACy5@HA-ZIF-8 group was more intense (Fig. S18B). The quantitative analysis of the fluorescence signals also followed the same trend (Fig. S18C). To further investigate the accumulation and penetration of nanoparticles in tumors, we took various tumors for frozen sections and observed the fluorescence signals with CLSM (Fig. S19). The siRNACy5@HA-ZIF-8 NPs displayed more aggregation and deeper penetration in tumors, which may be attributed to the binding of HA to the CD44 receptor on the cell surface. Above all, siRNACy5@HA-ZIF-8 NPs had excellent ability to target tumors, which laid the foundation for subsequent in vivo efficacy evaluation.
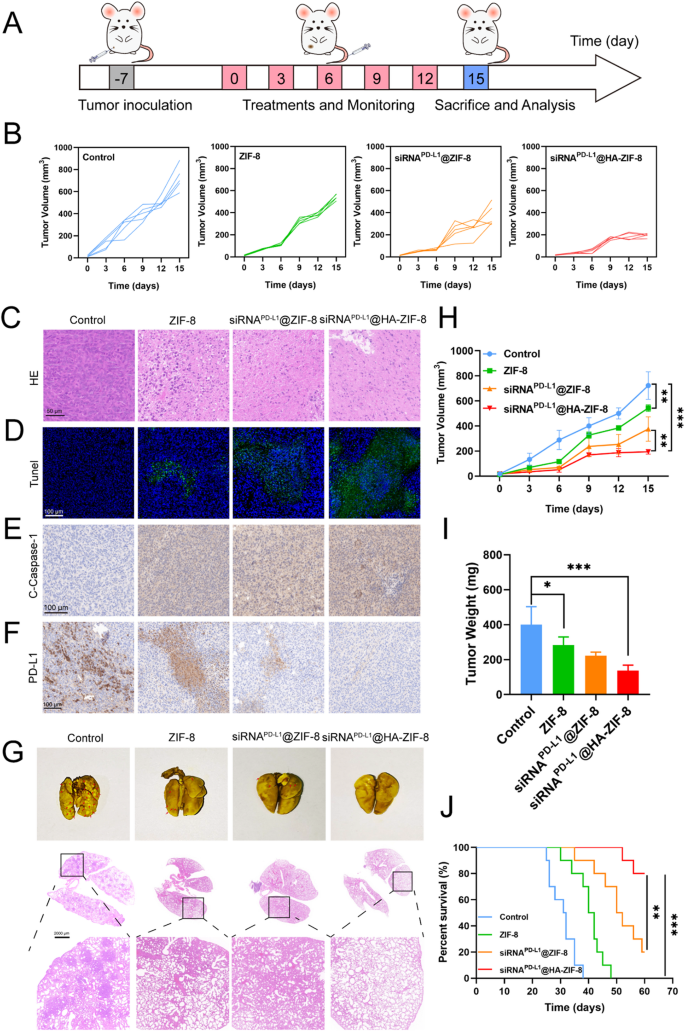
Subsequently, the in vivo anti-tumor effect was evaluated using the mouse model bearing 4T1 tumors. To achieve optimal anti-tumor efficacy while maintaining safety, we first set a series of dose gradients for evaluation (Fig. S20). With increasing doses administered, the inhibition of tumor growth exhibited a dose-dependent enhancement, where 5 mg/kg and 10 mg/kg presented similarly potent antitumor efficacy. However, at the 10 mg/kg dose, the treated mice demonstrated modest but statistically significant weight loss, indicating dose-limiting toxicity. Based on the optimal balance between antitumor efficacy and safety profile, a dose of 5 mg/kg was selected for subsequent investigations. To examine the antitumor efficacy of different preparations, tumor-bearing mice were randomly divided into 4 groups (n = 5): Control, ZIF-8, siRNAPD−L1@ZIF-8 and siRNAPD−L1@HA-ZIF-8. The treatment program followed the schedule in Fig. 5A. As shown in Fig. 5B and H, tumor growth was rapid in the Control group, whereas the ZIF-8 group displayed slight inhibition of tumor growth, suggesting the anti-tumor effect of pyroptosis induced by ZIF-8 NPs. After encapsulating siRNAPD−L1, the rate of tumor growth in siRNAPD−L1@ZIF-8 NPs group was further inhibited, indicating that the combination of pyroptosis and immune checkpoint inhibitors stimulated enhanced anti-tumor effects. Due to the targeting ability of HA, siRNAPD−L1@HA-ZIF-8 NPs showed superior controlled tumor growth. The excellent tumor suppression effect of siRNAPD−L1@HA-ZIF-8 NPs was also further proved by measuring the weight of the tumor (Fig. 5I) at the end of the treatment.
Besides, HE and Tunel immunostaining were performed for pathological analysis of tumor tissues. As shown in Fig. 5C, compared with Control group, the nanoparticle groups presented a large number of apoptotic cells, which were behaved as cell disruption and shrinkage, and absence of cell nuclei, with the most obvious in siRNAPD−L1@HA-ZIF-8 NPs group. In addition, Tunel immunostaining showed that tumors in siRNAPD−L1@HA-ZIF-8 NPs group displayed the highest proportion of apoptotic cells (Fig. 5D). To study the anti-tumor mechanism, immunohistochemistry (IHC) was carried out to examine the expression of C-Caspase-1 and PD-L1 in the tumors. C-Caspase-1 expression was significantly increased in tumors after treatment with siRNAPD−L1@HA-ZIF-8 NPs, signifying that it induced extensive pyroptosis (Fig. 5E). The elevated levels of Zn2+ in the tumors further confirmed the Zn2+ overload-induced pyroptosis (Fig. S21). Meanwhile, after treating with siRNAPD−L1@HA-ZIF-8 NPs, PD-L1 expression was significantly decreased, indicating effective PD-L1 expression inhibition (Fig. 5F). The above two IHC results demonstrated that siRNAPD−L1@HA-ZIF-8 NPs triggered intensive pyroptosis in the tumor and effectively silenced the expression of PD-L1 protein, which could be the mechanism for nanoparticles to exert anti-tumor effects.
To further verify the effect of nanoparticles on the survival of mice, another batch of mice bearing 4T1 tumors were treated with various preparations and their survival was monitored over a period of 60 days. As shown in Fig. 5J, all mice in the Control group died in less than 40 days, whereas the nanoparticles group prolonged the survival period of the mice to varying degrees, with more than half of the mice in siRNAPD−L1@HA-ZIF-8 NPs group still alive on day 60. The prolonged survival of mice may be attributed to the excellent anti-tumor effect of siRNAPD−L1@HA-ZIF-8 NPs.
Anti-tumor efficacy and anti-metastatic effect of siRNAPD−L1@HA-ZIF-8 NPs (A) Schematic illustration of the establishment of the 4T1 mouse model and in vivo therapy. (B) Tumor growth curves of mice in various groups during different treatments. HE (C) and Tunel (D) immunostaining of tumor tissues after various treatments. C-Caspase-1 (E) and PD-L1 (F) immunohistochemistry of tumor tissues after various treatments. (G) Photographs and HE staining of lung tissues after various treatments. (H) Average tumor growth curves during different treatments. (I) Mean tumor weight after treatment with different formulations. (J) Survival curves of mice treated with different formulations. Data were presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001
Anti-metastasis and in vivo biosafety evaluation
The susceptibility to lung metastasis is the most common phenomenon of triple-negative breast cancer, as well as the main causes of recurrence and resistance to eradication of breast cancer. After treatment, the lungs of the mice were extracted and fixed to observe the lung metastasis of tumors. Compared with the Control group, the lung tissues of nanoparticle-treated mice displayed significantly fewer tumor lung metastatic nodules, where the lung tissues of siRNAPD−L1@HA-ZIF-8 NPs group were almost invisible to the tumor nodules (Fig. 5G). To further explore the lung metastasis of tumor, the lung tissues were stained by HE for pathological analysis. According to the HE staining results, Control group appeared to contain abundant tumor cells with dense nucleus, whereas the nanoparticle groups presented reduced tumor cells. In particular, lung tissue sections of siRNAPD−L1@HA-ZIF-8 NPs group exhibited normal lung tissue morphology and virtually non-existent tumor cells. The superior anti-metastatic performance of siRNAPD−L1@HA-ZIF-8 NPs may result from the efficient anti-orthotopic tumor ability and systemic immune activation.
An ideal drug delivery system would expect that formulations are not only restricted to achieving superior therapeutic efficacy, but also emphasized on biosafety and biocompatibility, implying the avoidance of additional systemic toxicity and side effects. As shown in Fig. S22A, during the treatment period, there was no obvious change in the body weight of the mice in each group. Additionally, blood from mice was collected for haemocompatibility testing. No obvious hemolysis was observed for siRNAPD−L1@HA-ZIF-8 NPs at different concentrations (Fig. S23). Similarly, there was no significant difference in the hemolysis rate of siRNAPD−L1@HA-ZIF-8 NPs at different concentrations from that of saline (Fig. S24). At the end of treatments, the blood of mice was collected for serum biochemical tests, including ALT, AST, BUN and CRE. The results suggested that there was no significant difference in the serum biochemical indices between the nanoparticles group and Control group (Fig. S22B), implying no cause of hepatotoxicity and nephrotoxicity in mice. Moreover, major organs of mice (heart, liver, spleen and kidney) were extracted for HE staining after treatment. The sections showed no pathological damage in major organs in all groups of mice (Fig. S22C), which indicated that the nanoparticles had no obvious toxicity to the major organs of mice. The above results substantiated the outstanding biocompatibility and biosafety of siRNAPD−L1@HA-ZIF-8 NPs.
In vivo immune evaluation
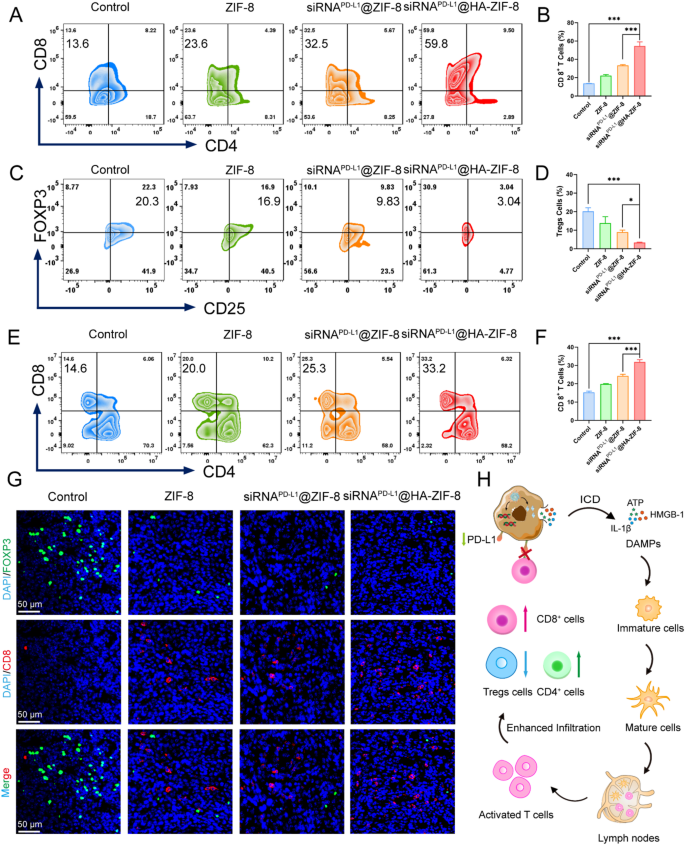
Since siRNAPD−L1@HA-ZIF-8 NPs demonstrated excellent anti-tumor effect and anti-metastatic ability, we further explored the mechanism of immune activation in mice. On the third day after various treatments, single-cell suspensions of tumors and spleens were dissected for flow cytometry analysis. The activation of the adaptive immune response is generally verified by examining the proportion of T helper cells (CD4+) and cytotoxic T lymphocytes (CTLs, CD8+) in the primary tumor. The results of flow cytometry analysis indicated that compared with the Control group, the proportion of CD8+ T cells in primary tumors of mice treated with nanoparticles was all increased (Fig. 6A), with the highest proportion of CD8+ T cells in siRNAPD−L1@HA-ZIF-8 NPs group, which was about 3.99-fold of that in the Control group (Fig. 6B). The high proportion of CD8+ T cells in primary tumors reflected an elevated T cells infiltration and potent activation of the immune response as expected. Regulatory T cells (Tregs), which are recruited by tumors to suppress cytotoxic T lymphocytes, cytokine production and immune responses, contribute significantly to the severe immunosuppressive tumor microenvironments of “cold” 4T1 cells. After treatment with siRNAPD−L1@HA-ZIF-8 NPs, the proportion of Tregs cells in primary tumors was considerably decreased than Control group (Fig. 6C and D), implying an attenuation of immunosuppression induced by pyroptosis. Since spleen acts as an important immune organ, we subsequently detected the proportion of CD8+ T cells in the spleens of each group of mice. As shown in Fig. 6E and F, the proportion of CD8+ T cells in the spleen of siRNAPD−L1@HA-ZIF-8 NPs group increased from 14.6% to 33.2% compared with that of Control group, which further confirmed the activated adaptive immunity in vivo. To further validate T cell infiltration and alteration of the immunosuppressive microenvironment in the tumor, immunofluorescence staining was subsequently performed for each tumor. As shown in Fig. 6G, the weakest FOXP3 green fluorescence was exhibited in the tumors of siRNAPD−L1@HA-ZIF-8 NPs group as compared to Control group, suggesting the presence of a minimal population of Tregs cells, which attenuated the immunosuppressive microenvironment of tumor tissue. In contrast, CD8 red fluorescent signals were expressed most strongly in siRNAPD−L1@HA-ZIF-8 NPs group, implying substantial CD8+ T cell infiltration and robust immune response activation in the tumor tissue. In addition, the strong CD19 and F4/80 fluorescence signals observed in immunofluorescence staining (Fig. S25 and Fig. S26) revealed a significant increase in the infiltration of B cells and macrophages within the tumors, thereby further confirming the robust immune response elicited. In summary, the mechanism of siRNAPD−L1@HA-ZIF-8 NPs exerting anti-tumor effects in vivo was shown in Fig. 6H as expected. In tumor cells, siRNAPD−L1@HA-ZIF-8 NPs underwent pH-sensitive cleavage and released Zn2+ ions and siRNAPD−L1. On the one hand, Zn2+ ions triggered pyroptosis and exposed cells to ICD. Subsequently, the released DAMPs promoted the maturation of DC cells, which activated and enhanced the infiltration of T lymphocytes in the tumor tissues. Meanwhile, the proportions of CD4+ and CD8+ T lymphocytes, B cells and macrophages were elevated and Tregs cells were reduced in the tumor, which implied the activation of innate and adaptive immunity and the depression of the immunosuppressive microenvironment. On the other hand, the released siRNAPD−L1 silenced the PD-L1 protein on the tumor surface and relieved the immune evasion of tumor cells against T cells. Overall, siRNAPD−L1@HA-ZIF-8 NPs synergistically enhanced the immune response in vivo and improved anti-tumor efficacy from the above two aspects.
In vivo immune stimulation effect. (A) Flow cytometry assay and (B) relative percentages of infiltrated CD4+ and CD8+ T cells in primary tumors after various treatments. (C) Flow cytometry assay and (D) proportions of Tregs (CD3+CD4+Foxp3+) in primary tumors after different treatments. (E) Flow cytometry assay and (F) relative percentages of CD4+ and CD8+ T cells in splenocytes after various treatments. (G) Immunofluorescence staining of CD8+ T cells (CD8) and Tregs cells (FOXP3) in primary tumors. (H) Schematic representation of enhanced immune activation mechanisms in vivo. Data were presented as mean ± SD. *P < 0.05, ***P < 0.001
Transcriptomic analysis
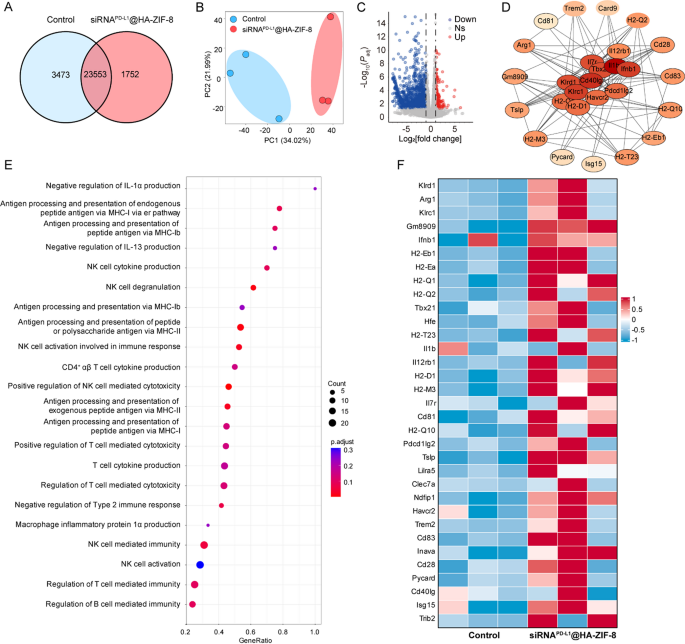
To explore the molecular mechanisms of anti-tumor immunotherapy more deeply, we performed transcriptome sequencing to analyze tumors treated with Control and siRNAPD−L1@HA-ZIF-8 NPs. As shown in Fig. 7A, the Venn diagram shows all expressed genes in each group. Principal component analysis (PCA) indicated significant differences between the two groups (Fig. 7B). As seen from the volcano plot (Fig. 7C), there were 1377 different genes in siRNAPD−L1@HA-ZIF-8 NPs group compared with Control group, of which 173 genes were up-regulated (red dots) and 1204 genes were down-regulated (blue dots). Gene Ontology (GO) enrichment analysis demonstrated that after siRNAPD−L1@HA-ZIF-8 NPs treatment, the differential genes were mainly enriched in inflammation-related signaling pathways and immune-related signaling pathways, including negative regulation of immunosuppressive molecules (IL-1α and IL-13), major histocompatibility complex (MHC) mediated antigen processing and presentation, NK cell cytokine production and mediated immunity and T cell cytokine production and mediated cytotoxicity (Fig. 7E). Subsequently, we analyzed the expression levels of genes related to inflammatory signaling and immunity and found that genes associated with the regulation of T cell-mediated immunity and production of pro-inflammatory factors (Fig. 7F), antigen processing and presentation via MHC-I (Fig. S27A), antigen processing and presentation via MHC-II (Fig. S27B), NK cell activation (Fig. S27C), and NK cell-mediated immunity (Fig. S27D) were significantly up-regulated in siRNAPD−L1@HA-ZIF-8 NPs group. Furthermore, protein-protein interaction (PPI) network revealed an interaction between genes of immune-related signaling pathways and inflammatory factor-related signaling pathways (Fig. 7D). Overall, siRNAPD−L1@HA-ZIF-8 NPs could promote the release of inflammatory cytokines and stimulate a vigorous inflammatory response through pyroptosis, thereby strengthening antigen presentation and initiating robust anti-tumor immune responses.
Transcriptomic analysis. (A) Venn diagram of all expressed genes. (B) Principal component analysis diagram of each group. (C) Volcanic plot of differentially expressed genes between Control and siRNAPD−L1@HA-ZIF-8 NPs groups. (D) Protein-protein interaction (PPI) network. (E) GO enrichment after siRNAPD−L1@HA-ZIF-8 NPs treatment. (F) Heat map analysis of genes involved in immune response and cytokine production