Synthesis and characterization of Ag-CeNP and Ag-CeNP@Cel
We synthesized Ag-CeNP@Cel according to the following steps (Fig. 1A). First, the hydrophobic uniform-size ultrasmall nanozyme core Ag-CeNP was constructed by a modified sol–gel reaction [22, 24]. Subsequently, owing to the strong ligand binding between oleylamine on the surface of the nanozyme core and the phospholipid of DSPE-mPEG2k, the hydrophobic Ag-CeNP were transferred from organic phase into the hydrophilic phase after encapsulated with phase transfer agent DSPE-mPEG2k [25]. The PEGylation improves water-dispersity, and biocompatibility and extends the half-lives in the bloodstream of nanozyme [26]. Finally, the anti-RA molecular Cel was loaded in the organic shell on the Ag-CeNP-PEG by van der Waals forces and organic macromolecules absorption [27]. The hydrodynamic diameter of Ag-CeNP-PEG based on dynamic light scattering (DLS) measurements was 6.77 ± 1.60 nm with a polydispersity index (PDI) of 0.37 ± 0.10 (Fig. 1B, Figure S1A), and the zeta potential was -0.01 ± 0.13 mV (Fig. 1C). After loaded Cel, the entrapment efficiency (EE%) of Cel was 87.07% and the hydrodynamic diameter of Ag-CeNP@Cel was increased to 8.53 ± 0.26 nm with a PDI of 0.24 ± 0.05 (Fig. 1D, Figure S1B), and the zeta potential was changed to -8.94 ± 0.73 mV (Fig. 1E). The changes in hydrodynamic diameter and zeta potential confirmed the successful loading of Cel in the organic shell of Ag-CeNP. The hydrodynamic diameter and relative turbidity of Ag-CeNP-PEG and Ag-CeNP@Cel did not show significant changes within 7 days, exhibiting that they do not agglomerate and have good colloidal stability in serum-containing media for over one week (Figure S2). As shown in Fig. 1F–G, the TEM images confirm that Ag-CeNP and Ag-CeNP-PEG maintain a highly facetted, crystalline spherical morphology with an ultrasmall core size of approximately 2.0 nm, which enhances catalytic efficiency and biological penetration. Energy-dispersive spectroscopy (EDS) verified the successful synthesis of Ag-CeNP, indicated by the necessary elements peak Ce, Ag, and O elements (Cu and C come from the carbon film) (Figure S3). Selected-area electron diffraction (SAED) patterns of Ag-CeNP showed halo-like patterns, the distinguish fringes confirmed the nanocrystalline nature (Fig. H). In addition, Fig. 1I showed the XRD patterns of Ag-CeNP, the diffraction peaks at 2θ of 28.4°, 33.0°, 47.4°, 56.3°, 59.1°, 68.9°, 77.3° and 88.6° can be assigned to the (111), (200), (220), (311), (222), (400), (331) and (422) lattice planes of the cubic fluorite structure of ceria nanoparticles (JCPDS file NO. 34–0394), while sharp reflections of 38.0°, 44.1°, and 64.4° can be attributed to (111), (200) and (220) lattice planes of the crystalline Ag peaks, which were well matched with cubic structure of Ag (JCPDS file NO. 87–0717) [28, 29]. The XPS survey spectrum indicated Ag-CeNP was composed of Ce (880–920 eV), O (~ 530 eV) and Ag (~ 360 eV) (Figure S4). High-resolution spectra of Ce3d and Ag3d were acquired to highlight nanozyme surface chemical character. The Ce3d envelope revealed characteristic mixed valence state of Ce3+ (884.9 and 902.8 eV) and Ce4+ (882.1,888.6, 898.0, 900.6, 907.2 and 916.4 eV) (Fig. 1J), this result is consistent with our previous research [23, 24]. Ag3d spectra consist of spin–orbit coupling Ag3d3/2 and Ag3d5/2 whose binding energies were 368.1 eV and 374.1 eV, respectively (Fig. 1K), which were excellent agreement with the reported AgNPs [30, 31].
Synthesis and characterization of silver-decorated ceria nanoparticle. A Schematic illustration of the synthetic process for Ag-CeNP, Ag-CeNP-PEG and Ag-CeNP@Cel. B Hydrodynamic size distribution of Ag-CeNP-PEG, determined by dynamic light scattering (DLS) in ddH2O (n = 3). C Apparent zeta potential distribution of Ag-CeNP-PEG in ddH2O (n = 3). D Hydrodynamic size distribution of Ag-CeNP@Cel, determined by DLS in ddH2O (n = 3). E Apparent zeta potential distribution of Ag-CeNP@Cel in ddH2O (n = 3). Representative high-resolution TEM image of hydrophobic nanozyme Ag-CeNP (F) and hydrophilic nanozyme Ag-CeNP-PEG (G). H The distinct lattice fringes in SAED patterns confirm the crystalline character of Ag-CeNP. I XRD patterns of Ag-CeNP indicate major peaks belonging to cubic phase CeNP (ICSD 55284) and minor peaks belonging to silver element (ICSD 44387). J Deconvoluted XPS spectrum of cerium Ce3d revealing the valence state and the corresponding binding energy peaks of Ce (III) (884.9 and 902.8 eV) and Ce (IV) (882.1, 888.6, 898.0, 900.6, 907.2 and 916.4 eV). K Deconvoluted XPS spectrum of silver Ag3d indicating Ag remaining in a metallic (Ag0) state. a.u., arbitrary units
Antioxidative activity of Ag-CeNP@Cel
To demonstrate the superiority of Ag-CeNP-PEG, we compared its ROS scavenging activity and macrophage polarization regulation with CeNP-PEG. The results showed that Ag-CeNP-PEG exhibited significantly higher DPPH free radical scavenging activity (Figure S5A), as well as enhanced superoxide dismutase (SOD)-like (Figure S5B) and peroxidase (POD)-like (Figure S5C) activities, compared to CeNP-PEG. In contrast, its hydroxyl radicals (•OH) scavenging activity (Figure S5D) and catalase (CAT)-like decomposition of hydrogen peroxide (H2O2) into O2 (Figure S5E) were comparable to those of CeNP-PEG. Cell-based experiments further confirmed that Ag-CeNP-PEG was more effective than CeNP-PEG in reducing intracellular ROS levels (Figure S6). Additionally, although both CeNP-PEG and Ag-CeNP-PEG reduced the M1-type polarization of BMDM, Ag-CeNP-PEG exhibited a stronger effect (Figure S7).
The hydroxyl radical antioxidant capacity (HORAC), SOD, and CAT of nanozyme were investigated by electron paramagnetic resonance (EPR) spectroscopy. EPR spectra showed the concentration-dependent scavenging activity of Ag-CeNP-PEG against •OH, superoxide anions (O2•⁻), and H2O2. As the concentration of Ag-CeNP-PEG increased, its capacity to neutralize •OH was enhanced (Fig. 2A), with a similar trend observed for O2•⁻ scavenging (Fig. 2B). In Fig. 2C, the concentration-dependent reduction in H2O2 further confirmed the CAT-mimetic activity of Ag-CeNP-PEG. Next, the radical scavenging activity of Ag-CeNP@Cel was also evaluated by the Superoxide Dismutase Assay Kit and Catalase Assay Kit. The results indicated that at the same nanozyme concentration, the SOD activity (units/μg) of Ag-CeNP@Cel was 0.4 times higher than Ag-CeNP-PEG. Additionally, at the same Cel concentration, the SOD activities (units/μg) of Ag-CeNP@Cel were 2.8 times higher than those of free Cel (Fig. 2D). Furthermore, at the same concentration, the CAT activity (units/μg) of Ag-CeNP@Cel was 0.2 times higher than that of Ag-CeNP-PEG and 0.7 times higher than that of free Cel (Fig. 2E). In addition, as shown in Figure S8, the reactive nitrogen species (RNS) elimination activity of Ag-CeNP@Cel was more pronounced than that of Ag-CeNP-PEG at the same nanozyme concentration and incubation time. The in vitro antioxidant activity of Ag-CeNP@Cel was further demonstrated using fluorescence microscopy and flow cytometry, employing the ROS probe Dihydrorhodamine 123 (DHR123). Flow cytometry analysis revealed that after 24 h of incubation with H2O2 (1.0 mmol/L), the ROS level in human umbilical endothelial cells (HUVEC) increased by 8.1 times. However, co-incubation with free Cel, Ag-CeNP-PEG, and Ag-CeNP@Cel significantly reduced by 77.7%, 84.2%, and 88.3%, respectively (Fig. 2F). The above results were validated by fluorescence microscopy, and intracellular ROS levels were significantly reduced after treatment with free Cel, Ag-CeNP-PEG, and Ag-CeNP@Cel (Fig. 2G), indicating that Ag-CeNP@Cel can alleviate oxidative stress induced by H2O2.
In vitro ROS scavenging activities of Ag-CeNP@Cel. EPR spectra demonstrated concentration-dependent scavenging activities of Ag-CeNP-PEG to •OH (A), O2•− (B), and H2O2 (C). D SOD-like activity of Ag-CeNP@Cel assessment by SOD activity assay kit (n = 3). E The catalase-like activity of Ag-CeNP@Cel assessment by CAT activity assay kit (n = 3). F Flow cytometric analysis evaluating the ROS levels in HUVEC after different treatments (n = 3). G Representative fluorescence microscopy images of HUVEC with different treatments and stained with ROS fluorescent probe DHR 123. Scar bar = 100 μm. The data are shown as the mean ± SD. One-way ANOVA was used for statistical analysis. (**p < 0.01, ***p < 0.001, ****p < 0.0001)
The cytotoxicity, uptake, and effects of Ag-CeNP@Cel on macrophage phenotype transitions
To test in vitro biocompatibility, the cytotoxicity of free Cel, Ag-CeNP-PEG, and Ag-CeNP@Cel was assessed on bone marrow-derived macrophages (BMDM) and HUVEC via MTT assay. After 24 h treatment, free Cel showed obvious cytotoxicity to BMDM at concentration higher than 0.16 μg/mL (Fig. 3A), and Ag-CeNP-PEG did not show cytotoxicity within a Ce concentration of 2 μg/mL (Fig. 3B), the cytotoxicity of Ag-CeNP@Cel was lower than that of free Cel (Fig. 3C), suggesting that loading Cel to Ag-CeNP-PEG reduced its cytotoxicity. The cytotoxicity of free Cel, Ag-CeNP-PEG, and Ag-CeNP@Cel against HUVEC exhibited a comparable pattern (Figure S9). As illustrated in Figure S10A, approximately 50% of the cells succumbed after incubation with 1 mmol/L H2O2 for 24 h. Thus, this concentration was selected for modeling oxidative damage cell models. The free Cel, Ag-CeNP-PEG, and Ag-CeNP@Cel were observed to enhance the viability of H2O2-stimulated HUVEC, with the Ag-CeNP@Cel demonstrating the most pronounced effect (Figure S10B). To further study the anti-apoptotic effect of Ag-CeNP@Cel, apoptotic cells were quantified using the Annexin V-FITC/PI assay. Total apoptotic cells were 19.19 ± 0.95% in the H2O2-stimulated model group, it decreased to 16.29 ± 0.22%, 13.53 ± 0.25%, and 12.88 ± 0.18% after treatment with free Cel, Ag-CeNP-PEG, and Ag-CeNP@Cel, which indicated that the anti-apoptosis effect of Ag-CeNP@Cel more significant than free Cel and Ag-CeNP-PEG on H2O2-stimulated cells model (Fig. 3D-E). The cellular uptake of nanozyme was explored by monitoring the Ag-CeNP-PEG labeled with Nile Red. Normal and injured HUVEC were used as models. Flow cytometry revealed that cellular uptake was as early as 30 min after the addition of Ag-CeNP-PEG and internalized by cells in a time-dependent manner. Moreover, the nanoparticle uptake efficiency of H2O2-injured HUVEC was higher than that of normal HUVEC (Fig. 3F), indicating that nanozyme was more likely to enter cells damaged by oxidative stress to exert protective effects. BMDM was used to determine the capability of Ag-CeNP@Cel for reprogramming macrophages (Fig. 3G). Flow cytometry showed that CD86, a marker of pro-inflammatory macrophages, was pronounced in LPS + IFN-γ stimulated BMDM, while treatment with free Cel, Ag-CeNP-PEG, and Ag-CeNP@Cel decreased CD86 expression by 25.0%, 74.3% and 80.8%, respectively (Figure S11, Fig. 3H). Interestingly, the expression of the anti-inflammatory macrophage marker CD206, was decreased in LPS + IFN-γ stimulated BMDM, after free Cel, Ag-CeNP-PEG, and Ag-CeNP@Cel treatment, increased by 25.6%, 57.5%, and 103.2%, respectively (Figure S12, Fig. 3I). The ratio of CD86/CD206 was markedly diminished by treatment with free Cel, Ag-CeNP-PEG, and Ag-CeNP@Cel, with Ag-CeNP@Cel exhibiting the most pronounced effect (Fig. 3J). Collectively, these results suggested that Ag-CeNP@Cel effectively repolarizes pro-inflammatory macrophages to anti-inflammatory macrophages, thereby exerting an anti-inflammatory effect.
In vitro cytotoxicity, uptake, and macrophage phenotype transition test of Ag-CeNP@Cel. The viability of BMDM post-treatment of free Cel (A), Ag-CeNP-PEG (B), and Ag-CeNP@Cel (C) for 24 h (n = 6). D Flow cytometry based on Annexin V-FITC/PI apoptosis detection kit post-stimulated by 1 mM H2O2 and then treatment of free Cel, Ag-CeNP-PEG, or Ag-CeNP@Cel for 24 h. E The quantification of apoptosis of HUVEC post varied treatment (n = 3). F Quantitative analysis of the in vitro cellular uptake of Nile Red-labelled Ag-CeNP-PEG in normal HUVEC and H2O2 stimulated HUVEC detected by flow cytometry (n = 3). G Schematic diagram of the experimental setup to investigate the effect of Ag-CeNP@Cel modulate the phenotype of macrophage. Quantitative analysis of M1 macrophage markers CD86 (H) and M2 macrophage marker CD206 (I) by flow cytometry treated with various treatments (n = 3). J The ratio of M1 and M2 macrophages in different treatment groups (n = 3). The data are shown as the mean ± SD. One-way ANOVA was used for statistical analysis. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001)
In vivo targetability of Ag-CeNP-PEG
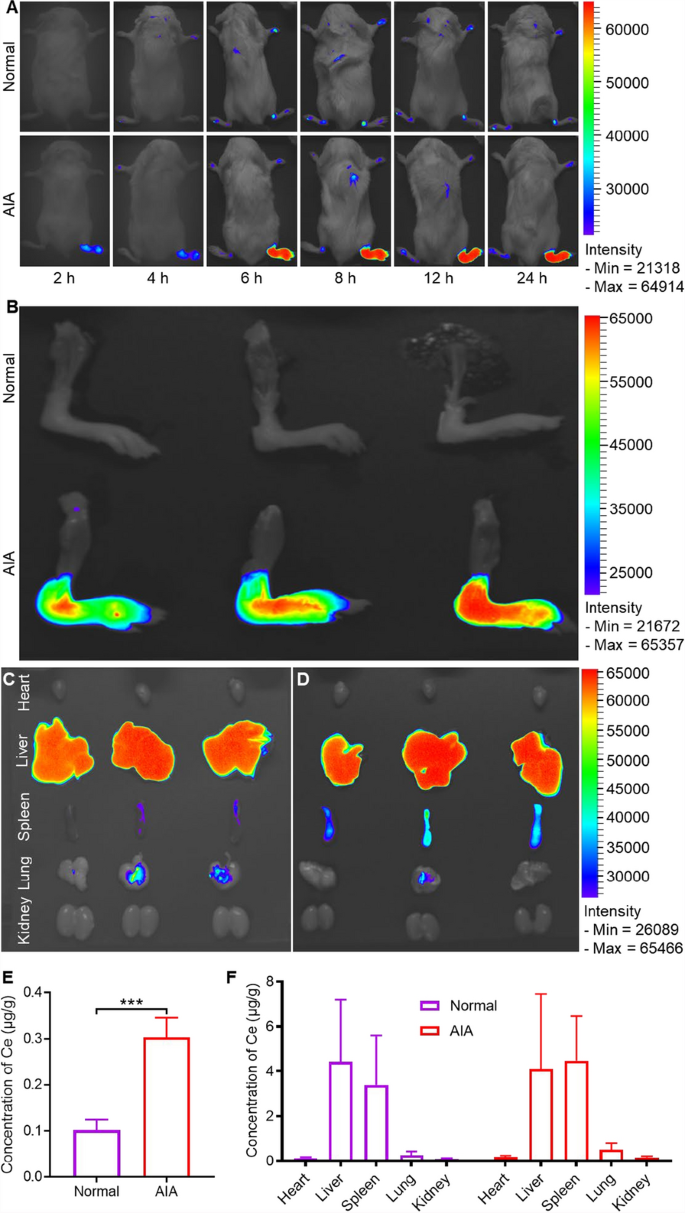
The in vivo targetability of Ag-CeNP-PEG was investigated using adjuvant-induced arthritis (AIA) murine model, which was developed through intradermal injection of complete Freund’s adjuvant into the right hind paws [32]. This model mimics key pathological features of human RA, including chronic inflammation, cartilage degradation, and bone destruction. To assess the accumulation of Ag-CeNP@Cel in inflamed joints, Did-labeled Ag-CeNP-PEG and free Ag-CeNP-PEG were administered intravenously to both normal and AIA mice. The distribution was tracked using an optical imaging system and quantified by inductively coupled plasma mass spectrometer (ICP-MS). As shown in Fig. 4A, no significant fluorescence was detected in the paws of normal mice during the 24 h observation period. However, the inflamed paws of AIA model mice exhibited much stronger fluorescence signals compared to the contralateral non-inflamed paws and the paws of normal mice. Notably, the fluorescence intensity in the inflamed paws increased gradually over time, peaking at 8 h post-injection and remaining strong for at least 24 h. Ex vivo imaging of the joints at 24 h post-injection further confirmed these results. While no fluorescence was detected in the non-inflamed legs of normal mice, the inflamed legs of AIA mice displayed strong fluorescence (Fig. 4B). Semi-quantitative analysis of fluorescence intensity revealed that the inflamed legs of AIA mice had approximately fourfold higher fluorescence compared to the non-inflamed legs of normal mice (Figure S13). Ex vivo imaging of organs revealed that a significant number of Ag-CeNP-PEG nanoparticles accumulated in the liver and spleen (Fig. 4C, D), likely due to capture by the reticuloendothelial system (RES). Additionally, a smaller amount of nanoparticles was detected in the lungs (Fig. 4C, D). Consistent with the optical imaging results, quantitative data from ICP-MS demonstrated that the Ce concentration in inflamed joints was 0.30 ± 0.04 μg/g, which is three times higher than in normal joints (0.10 ± 0.02 μg/g) (Fig. 4E). After intravenous injection, the majority of nanoparticles were sequestered by the RES, primarily accumulating in the liver and spleen (Fig. 4F). These findings highlight the in vivo targeting ability of Ag-CeNP@Cel, demonstrating its selective accumulation in inflamed joints. This passive accumulation of nanoparticles in inflamed areas can be attributed to the “ELVIS” effect, as previously established in related studies [4, 33].
High arthritis-targeting efficiency of Ag-CeNP-PEG. A Real-time near-infrared fluorescence imaging of Did-labelled Ag-CeNP-PEG at selected time points post intravenous injection in normal and AIA mice. B Ex vivo fluorescent imaging of Did-labelled Ag-CeNP-PEG in hind limbs of normal and AIA mice 24 h post-injection of Ag-CeNP-PEG. Ex vivo fluorescent imaging of Did-labelled Ag-CeNP-PEG in heart, liver, spleen, lung, and kidney of normal (C) and AIA mice (D) at 24 h post-injection. E ICP-MS analysis of Ag-CeNP-PEG accumulation in normal and RA-affected joints (n = 4). F ICP-MS analysis of Ag-CeNP-PEG distribution in the heart, liver, spleen, lungs, and kidneys of normal and RA mice (n = 4). The data are shown as the mean ± SD. One-way ANOVA was used for statistical analysis. (***p < 0.001)
In vivo therapeutic efficacy of Ag-CeNP@Cel on AIA mice
The in vivo therapeutic efficacy of Ag-CeNP@Cel was evaluated in AIA mice. Figure 5A illustrates the temporal progression of the RA model establishment and treatment. At the end of the therapeutic process, the mean body weights of the model group, the Ag-CeNP-PEG group, the free Cel group, and the Ag-CeNP@Cel group at the end of the experiment were 106.5%, 106.7%, 89.8%, and 98.3% of their respective baseline weights, respectively. These findings suggest that free Cel may possess a higher degree of toxicity, whereas the encapsulation of Cel within nanozyme may have effectively mitigated its toxicity (Fig. 5B). While Ag-CeNP-PEG was ineffective in reducing the arthritis index and paw thickness in AIA mice, free Cel showed some therapeutic effects. In contrast, Ag-CeNP@Cel significantly reduced the arthritis index and alleviated ankle joint swelling compared to both free Cel and Ag-CeNP-PEG (Fig. 5C, D). As shown in Fig. 5E, compared to healthy mice, mice in the model group experienced severe joint swelling, while mice treated with free Cel and Ag-CeNP-PEG showed moderate improvement, with reduced swelling. The Ag-CeNP@Cel group, however, displayed significant reductions in joint swelling. Micro-CT scans confirmed these findings, with severe bone erosion in the model group and some reduction in bone damage in the free Cel and Ag-CeNP-PEG groups. Notably, the Ag-CeNP@Cel group exhibited minimal bone erosion and smooth bone surfaces (Fig. 5F, Figure S14), indicating substantial improvement in the progression of bone damage. Several key histomorphometric indices based on micro-CT scans were also evaluated, including trabecular bone surface (Tb.BS), trabecular bone volume (Tb.BV), trabecular bone mineral content (Tb.BMC), trabecular thickness (Tb.Th), trabecular bone mineral density (Tb.BMD), and TbCav.CT_Value. In the AIA mouse model, Tb.BS, Tb.BV, and Tb.BMC was significantly increased, while Tb.BMD, Tb.Th and TbCav.CT_Value was decreased compared to the control group. However, treatment with free Cel, Ag-CeNP-PEG, and Ag-CeNP@Cel effectively decreased Tb.BS, Tb.BV, and Tb.BMC while increasing Tb.BMD and Tb.Th. Notably, the Ag-CeNP@Cel group recovered to levels closest to the control group (Fig. 5G-L), demonstrating its efficacy in halting and repairing bone erosion. Overall, these results suggest that Ag-CeNP@Cel can effectively terminate bone erosion progression and enhance its repair, with Cel-loaded Ag-CeNP-PEG significantly augmenting therapeutic outcomes.
In vivo anti-arthritis efficacy of Ag-CeNP@Cel. A The schematic illustration of the experimental timeline for Ag-CeNP@Cel treatment for AIA mice. The variation of body weight percentage (B), arthritis index (C), and paw thickness (D) of AIA mice during the treatment period (n = 6). Representative images of right hind paws (E) and three-dimensional reconstruction of micro-CT of the right hind paws (F) at the endpoint of the experiment from each group. Quantitative bone tissue analysis parameters of Tb.BS (G), Tb.BV (H), Tb.BMC (I), Tb.Th (J), Tb.BMD (K) and TbCav.CT_Value (L), as calculated from the micro-CT scanning results (n = 4). Scale bar: 5 mm in E, and 1 mm in F. The data are shown as the mean ± SD. One-way ANOVA was used for statistical analysis. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001)
Anti-inflammatory mechanism of Ag-CeNP@Cel in RA
The anti-inflammatory mechanism of Ag-CeNP@Cel in RA was investigated through a series of experiments. The relative abundance of pro-inflammatory (M1) macrophages was elevated in the inflamed joints of AIA model mice, but this was reduced following treatment with the various formulations (Figure S15, Fig. 6A), with Ag-CeNP@Cel showing the most effective shift from a pro-inflammatory (M1) to an anti-inflammatory (M2) macrophage phenotype (Figure S16, Fig. 6B). The percentage of Foxp3+ CD25+ regulatory T cells (Tregs), which were reduced in the AIA model group, was significantly restored with treatment, with Ag-CeNP@Cel showing a particularly strong recovery effect (Figure S17, Fig. 6C). Additionally, untreated AIA mice exhibited the highest percentage of CD31+ cells, indicating the involvement of angiogenesis in RA progression and highlighting the selective accumulation of Ag-CeNP@Cel in the inflamed joint (Figure S18, Fig. 6D). Vimentin-positive synovial fibroblasts, which are responsible for synovial hyperplasia and joint destruction in RA, were significantly increased in the AIA model group but were effectively reduced by Ag-CeNP@Cel treatment, showing the most pronounced decrease among all treatments (Figure S19, Fig. 6E). Cytokine analysis revealed that the AIA model mice had the highest concentrations of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) and the lowest levels of anti-inflammatory cytokine (IL-4). Treatment with Ag-CeNP@Cel significantly reduced pro-inflammatory cytokines while increasing anti-inflammatory cytokine levels in both serum (Fig. 6F) and inflamed joints (Fig. 6G), confirming its ability to restore immune regulation in AIA.
Immunomodulatory, angiogenesis inhibition and anti-inflammatory effect of Ag-CeNP@Cel. Flow cytometric analysis of CD86+ M1 macrophages (A), CD206+ M2 macrophages (B), Foxp3+ CD25+ Treg cells (C), CD31+ synovial endothelial cells (D) and vimentin+ synovial fibroblasts (E) isolated from inflamed joints of AIA mice at the end point of the experiment from each group (n = 3). ELISA analyses of inflammatory cytokine (IL-1β, IL-6, and TNF-α) and anti-inflammatory cytokine (IL-4) levels in the serum (F) and inflamed joints (G) of AIA mice at the end point of the experiment from each group (n = 3). The data are shown as the mean ± SD. One-way ANOVA was used for statistical analysis. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001)